INTRODUCTION
Fixed orthodontic appliances increase the incidence of white spot lesions (WSLs) on the facial surface of teeth because the appliances provide spaces for plaque retention.
1,
2,
3 After the removal of the orthodontic brackets or bands, the WSLs can be arrested because of changes in the oral environment. In some cases, however, the WSLs still remain as whitish opacities, causing an esthetic issue.
4,
5,
6 The enamel opacities can be described by an optical phenomenon, which is dependent on the pore volume of the lesion body of the WSLs. During demineralization of the enamel, the pore volume of the WSLs increases, and hence, the refractive index (RI) of the lesions can be altered by light scattering. The resulting difference in the refractive indices between the sound enamel and the caries lesion is observed as WSLs, which can be visually distinguished from the surrounding sound enamel.
7
Resin infiltration is a treatment for early caries lesions, such as WSLs. Originally, the aim of this treatment was to prevent further progression of early caries lesions by occluding pores of the lesion body, which acts as a cariogenic acid pathway. However, more attention has been paid to the additional effects of resin infiltration in terms of improving the esthetic appearance of the WSLs.
6,
7,
8 Recently, a very low viscosity resin, referred to as "infiltrant," optimized for resin infiltration has been developed. This material acts by occluding the pores of the lesion body by capillary forces,
9,
10 and when the pores are filled with the infiltrant, the infiltrated WSLs appear to be similar to the surrounding sound enamel because of the negligible difference in the refractive indices between the sound enamel (RI = 1.62) and the infiltrant (RI = 1.46).
5,
6,
7 Therefore, if the pores of the lesion body can be completely occluded with the infiltrant, the progression of the WSLs can be prevented. Further, the esthetic issues can also be resolved. For the complete occlusion of the lesion bodies with the infiltrant, it is necessary for the surface layer of the WSLs to be porous. This is because the relatively intact surface layers of the WSLs hamper the penetration of the infiltrant. Thus, in order to completely remove the surface layer and expose the lesion body of the WSLs, several previous studies and commercially available infiltrant kits (ICON®, DMG, Hamburg, Germany) have recommended the use of 15% hydrochloric acid gel (HCl) for 120 seconds.
11,
12 However, the WSLs often become arrested after the orthodontic treatment, thus making the complete removal of the surface layers by the recommended method difficult. The reason is that the arrested WSLs have thicker surface layers compared to the active lesions. Since it was hard to predict the masking effect of the arrested facial WSLs using the recommended method, previous studies attempted to perform the etching step using HCl repeatedly as a pretreatment.
5,
6 However, HCl is an extremely aggressive acid, which requires to be cautiously applied.
13 According to an earlier study,
14 when a microabrasion compound containing 10% HCl was contacted with soft tissues for more than 30 seconds, considerable amount of ulceration resulted, which took more than 24 hours to heal. Therefore, since the WSLs on the facial surface are usually located close to the gingival margin after orthodontic treatment,
15,
16 unintended gingival damages might result because of the application of a strong acid like 15% HCl. Hence, 15% HCl gel should be used in the presence of a rubber dam. However, during some orthodontic treatments, it is not possible to completely isolate the gingiva by using a rubber dam because of the fixed retainer or orthodontic appliances, and hence, additional isolation is needed.
5,
6 Hence, there is a necessity to develop a safer surface treatment method, which uses a weaker acid and reduced contact times.
Phosphoric acid (H
3PO
4) was introduced in dentistry in order to enhance resin retention, and has been widely used because of its safety.
17 In this study, we considered the use of H
3PO
4 for etching before the resin-infiltrant treatment. In addition, since the demineralized tooth surface is more prone to abrasion through mechanical friction, we attempted to combine the chemical effects of the acids with some mechanical effects originating from the mode of application to enhance the penetration of the resin infiltrant and the consequent masking effect on the WSLs.
18
Therefore, the first aim of this in vitro study was to investigate if applying 37% H3PO4 with a mechanical technique can be an alternative pretreatment method for resin infiltration. In this study, we also attempted to evaluate the penetration effects of the infiltrant by various pretreatment methods.
MATERIALS AND METHODS
Specimen preparation
Enamel specimens were obtained from sound bovine incisors. Specimens (5 × 3 mm) were sectioned with a low-speed hand-piece (Lasung Medice Co. Ltd., Incheon, Korea). The specimens were embedded in an acrylic resin (Ortho-Jet; Lang Dental Mfg., Wheeling, IL, USA) and sanded to flatness using 600-1,200 grit silicon carbide papers (Allied High Tech Products Inc., Comptom, CA, USA). Before making caries lesions, an area of 3 × 1 mm of each specimen was covered with acid-resistant nail varnish to prevent damage. Each specimen was then immersed in 40 mL of a demineralizing solution for 50 days.
19 Next, at the baseline, a portion of every individual caries lesion was additionally covered with nail varnish leaving a 3 × 3 mm area exposed. Fifty two specimens were randomly divided into four groups (n = 13 each). Ten specimens were used to evaluate the thicknesses of the removed surface layers and three specimens were used to evaluate the micromorphological changes in the lesion surfaces of each group.
Additionally, a total of ten specimens (5 × 3 mm) with 3 × 2 mm lesions at the center were created for evaluating the infiltrated areas. The specimens were cut perpendicular to the lesion surface with a microtome (TechCut 4™; Allied High Tech Products Inc.), yielding two halves for each lesion. After varnishing the cut surfaces, the paired lesion halves were allocated to either one of the two groups (n = 10 each).
Procedure for removing the surface layer
As a positive control, commercially available 15% HCl gel included in the ICON® kit was applied for 120 seconds. These samples were termed as the 15% HCl only group. Commercially available 37% H3PO4 gel (ETCH-37™; Bisco, Schaumburg, IL, USA) was applied for 30 seconds to three groups using three respective methods, and the samples were termed as follows: the 37% H3PO4 only group, the 37% H3PO4 sponge (ICON® kit) group, and the 37% H3PO4 brush (CavityShield® kit; 3M ESPE, St. Paul, MN, USA) group. In the case of the "only acid" group, the acid gel was simple placed on the sample. When a sponge or brush applicator was used, the acid gel was gently rubbed with applicators during the application time. The application force was less than 10 g, as determined by an electronic scale. All the processes were performed by a single trained researcher.
Observation of micro-morphological changes of surface layer
After acid treatment, the micromorphological changes in the lesion surfaces were observed using a field-emission scanning electron microscope (FE SEM-S800; Hitachi, Tokyo, Japan) at ×5,000 magnification.
Procedure for resin infiltration
The different pretreatments were applied on the surface layers of the two groups of samples. As a positive control, 15% HCl gel was applied only for 120 seconds. In the experimental group, 37% H3PO4 gel was gently applied with a brush for 30 seconds. Subsequently, after rinsing for 30 seconds, the infiltration procedure was performed according to the manufacturer's instructions (ICON®) on samples in both groups.
Evaluation
The specimens were cut perpendicular to the treated surface using a microtome. The nail varnish was carefully removed from all specimens. Subsequently, all the specimens were immersed in 50% ethanol containing 100 µM sodium fluorescein (NaFl; Sigma Aldrich, Steinheim, Germany) for 10 minutes.
20 After washing with deionized water for 3 minutes, the sectioned surfaces were evaluated using a confocal laser scanning microscope (CLSM; LSM710, Carl Zeiss, Oberkochen, Germany) in the fluorescence mode at magnifications of ×100 and ×400. The infiltrated specimens were specifically observed using the tile scan, which provided continuous images of the whole sectioned surface. The wavelengths of the excitation light were 488 and 543 nm. Also, 493-634 nm and 500-550 nm band pass filters were used for emission. Images were recorded with a lateral resolution of 1,024 × 1,024 pixels and dimensions of 213.13 × 213.13 µm
2. Lesion depths and the removed thicknesses of the surface layer were measured at three defined points using the proprietary analysis program associated with the CLSM equipment (ZEN 2009 LE software; Carl Zeiss). Relative lesion area (LA) and infiltrated area (IA) were evaluated using an image analysis system (Image-Pro Plus version 6.0; Media Cybernetics, Silver Spring, MD, USA). Finally, the percentage of the infiltrated area (IA%) was calculated (as IA% = IA/LA × 100).
Statistical analysis
The normal distribution and homogeneity of variance were checked for all data (by the Shapiro-Wilk and Levene tests, respectively). The thicknesses of the removed surface layers showed a normal distribution; however, the values did not show any homogeneity in intergroup variance. Therefore, the robust analysis of variance (ANOVA; Welch test) was used to compare the thickness of the removed surface layers, and the Games-Howell post-hoc test was used for subgroup comparisons. Also, for infiltrated areas, differences in the lesion depths and the IA% were compared using t-tests. Statistical analysis was performed using PASW Statistics ver. 18.0 for Windows (IBM Co., Armonk, NY, USA). The level of significance was set at 5% for the tests.
RESULTS
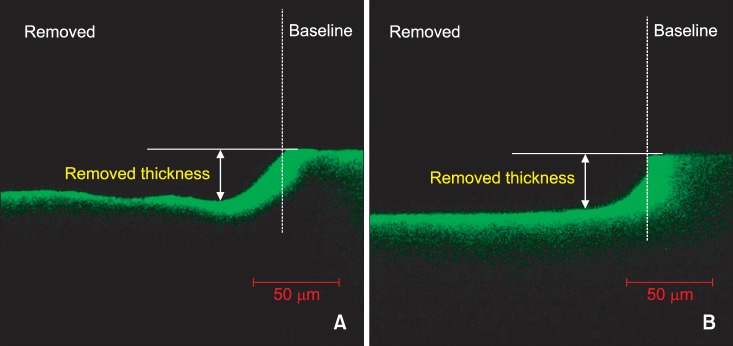
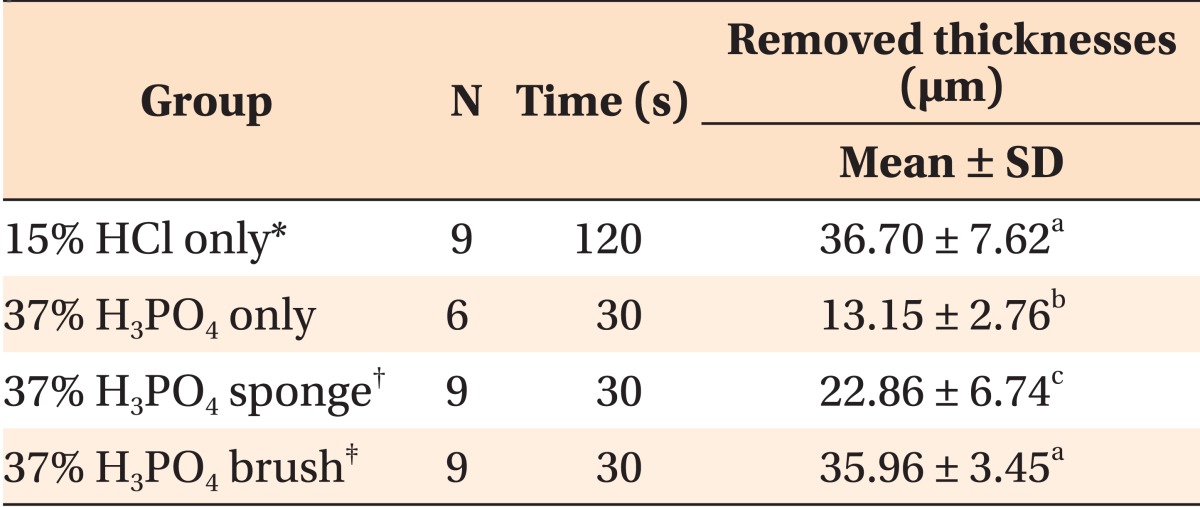
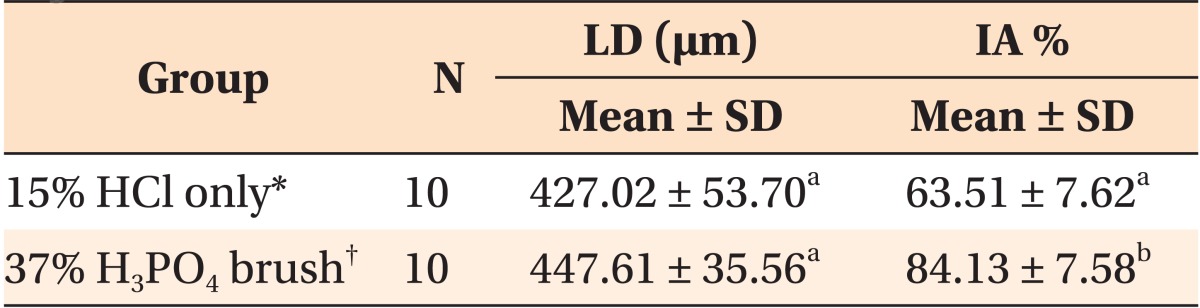
The thicknesses of the removed surface layers are presented in
Table 1. Thirty three specimens were subjected to CLSM analysis except for seven specimens damaged during the preparation. No significant difference could be observed in the removed thicknesses between the 15% HCl only group (positive control) and the 37% H
3PO
4 brush group (
p = 0.993). The removed surface layers were found to be adjacent to the baseline covered with acid resistant nail varnish (
Figure 1). However, in the 37% H
3PO
4 only group and 37% H
3PO
4 sponge group, the removed thicknesses were lesser than that observed in the case of positive control.
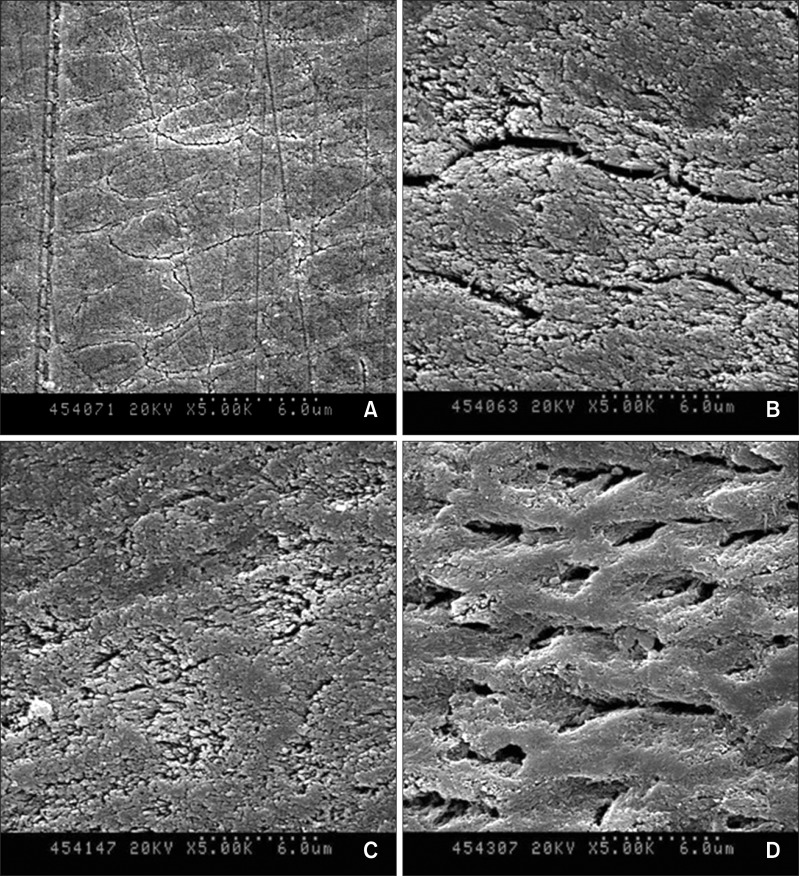
After acid treatment, the micromorphological changes in the lesion surfaces were observed by SEM (
Figure 2). A large increase in the pore volume was observed in the 37% H
3PO
4 brush group over the lesion surfaces (
Figure 2D) when compared to that observed in the positive control group specimens (
Figure 2B). The pore volume only marginally increased in the case of the 37% H
3PO
4 sponge group specimens in comparison to the specimens in the positive control and 37% H
3PO
4 brush group (
Figure 2).
The positive control group and the 37% H
3PO
4 brush showed considerably different IA% values (
p < 0.001,
Table 2). Specimens pretreated with 37% H
3PO
4 brush for 30 seconds showed a significantly higher mean IA% (84.13 ± 7.58%) than the positive control group (63.51 ± 7.62%).
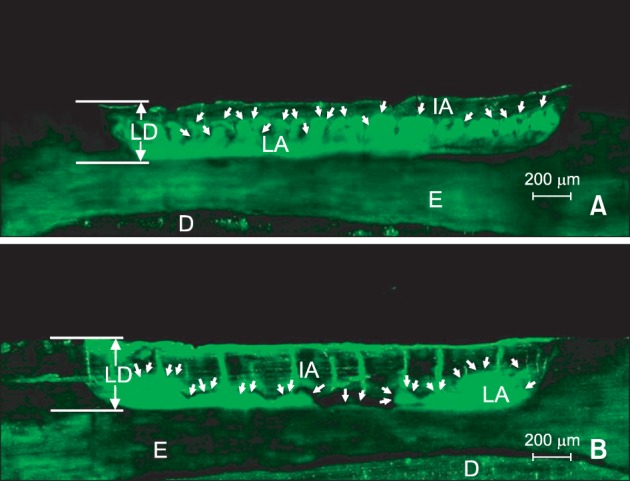
In the CLSM images, the non-infiltrated areas in the lesion body appeared fluorescent green because of staining with NaFl (
Figure 3), whereas the infiltrated areas filled with the infiltrant and the non-porous structures like sound enamel appeared dark. Deep infiltrant penetration was observed in the 37% H
3PO
4 brush group (using treatment time of 30 seconds) (
Figure 3B) in comparison to the positive control group (
Figure 3A).
DISCUSSION
In the present study, the use of 37% H3PO4 in conjunction with an applicator such as brush or sponge was found to increase the pore volume of the surface layer of caries lesions. When 37% H3PO4 was used with a brush, its surface layer removing effect was similar to that of the positive control (15% HCl only group); however, the pore volume was higher than the positive control group. Furthermore, the application time was reduced from 120 to 30 seconds. In order to evaluate the efficacy of the pretreatment, we treated the WSLs with the infiltrant and compared the IA% between the two groups. Our observations indicate that the infiltrant penetrated deeper and more evenly in the 37% H3PO4 brush group than in the positive control group.
The amounts of the surface layers removed were dependent on the application methods. When a brush was used, the removed depth was 2.7 times greater than that observed in the case of the 37% H
3PO
4 only group, and in the case of the sponge applicator, the removed depth also increased by 1.7 times. Combining the chemical and mechanical methods significantly increased the amount of the surface layers removed in comparison to that removed with the chemical method alone. It is well known that an acid-softened tooth surface is more prone to abrasion. Brushing of the tooth immediately after an erosive attack has been shown to cause 5-6 times more enamel surface loss than that caused by acid attack alone.
18,
21 This effect was more noticeable when the brush applicator rather than the sponge applicator was used. Also, as shown in the SEM images, the sponge applicator could not significantly increase the porosities of the surface layers. From these observations, it can be assumed that the mechanical friction caused by the sponge applicator was probably less than that caused by the brush applicator. Because the brushes consist of a number of filaments and have rough surfaces, they exert a higher friction force than the sponge applicator.
It is often challenging to control the amount of tooth surface removed by mechanical removal.
12,
22 However, with the brush applicator, to the amount removed was fairly simple to control. Since the brush used in this study was very flexible, it was easy to bend the brush even under slight pressure and hence, the applied forces were consistently maintained, which resulted in obtaining identical depths of removed tissue. This could be confirmed from the lower standard deviation of the thicknesses of the removed surface layers that was about half of the value observed for the positive control. In addition, the smear layers, which could be generated during the mechanical removal of the enamel, were not observed by SEM in the 37% H
3PO
4 brush group because the acid was strong enough to dissolve and remove the smear layers.
23
According to the SEM images, the removed surfaces of all the groups tended to be more porous than the unetched lesion surface; however, the etching patterns and the amounts by which the pore volumes increased were different for each group. In this study, the positive control produced clefts and grooves on the lesion surfaces, resulting in rough surfaces. When 37% H
3PO
4 was applied with the sponge applicator for 30 seconds, the lesion surfaces not only produced ill-defined clefts and grooves, but also showed the absence of any evidence of porous surfaces sufficient for resin infiltration. However, when the lesion surfaces were applied with 37% H
3PO
4 using a brush for 30 seconds, relatively smooth surfaces with numerous holes were produced. In adhesive dentistry, etching patterns with rough surfaces are considered to be most suitable for mechanical retention in resin restoration.
24 However, smooth etching patterns produced by 37% H
3PO
4 using the brush would be more advantageous for resin infiltration because when the infiltrant penetrates the lesion body, there is nothing left on the top of the surface unlike restoration resins or fissure sealants, which end up covering the tooth surfaces. Furthermore, the smooth surface is often more resistant to demineralization and bacterial accumulation.
According to a previous study,
12 in natural lesions of human teeth eroded with 15% HCl for 120 seconds, the removed depth ranges from 20 to 45 µm. However, in the lesions eroded with 37% H
3PO
4 for 30 seconds, the removed depth ranged from 0 to 20 µm. In this study, although artificial WSLs of bovine teeth, which are known to have surface layers thinner than those of natural lesions, were used instead of the natural WSLs of human teeth, the results of the study shows that the removed depths of the surface layer were relatively similar to those of the previous study. For example, in the lesions eroded with 15% HCl for 120 seconds, the removed depths ranged approximately from 22.75 to 45.82 µm, and in the lesions eroded with 37% H
3PO
4 only for 30 seconds, the range was 8.8-17.15 µm. The similar results can be ascribed to the unique surface protective agent we used during the process of artificial demineralization,
19 which helped the production of artificial caries lesions with surface layers relatively similar to those of natural teeth.
On the other hand, to evaluate the infiltrant penetration, it is more desirable to consider the area measurements rather than the depth measurements because the former contain more information about the infiltrant penetration. When the infiltrated depths were measured from certain points at regular intervals, the values could have been changed according to the starting points because the infiltrated depths were not consistent even in one tooth (
Figure 3). According to a recent study, when 15% HCl was applied with a microbrush for 120 seconds, the IA% was approximately 80%.
25 According to the results of our study, the IA% was 63.51 ± 7.62% when 15% HCl was applied without any applicators for 120 seconds. The lower value obtained in the present study may be attributed to the fact that we followed the manufacturer's instructions for the application of 15% HCl, which resulted in only chemical effects. However, the previous study combined chemical and mechanical effects to completely remove the surface layer. The application of 15% HCl with a microbrush is similar to the experiments reported in the present study that use applicators, because obtaining consistent results with inactive lesions on which 15% HCl gel was applied (without applicators) was challenging.
5,
6,
25 Therefore, in our study, applicators were used. The results of our study show that the IA% was about 84.13% when the lesions were removed with 37% H
3PO
4 by using the brush for 30 seconds. These results are similar to the values reported in the stated recent study, in which HCl was applied with a microbrush for 120 seconds.
Similar to previous studies,
9,
12 the present study also demonstrated that the increased pore volume of the surface layer plays an important role in improving the efficacy of resin infiltration. In addition, it was proven that although the thicknesses of the removed surface layers were similar in the two groups, the porosities were higher in the 37% H
3PO
4 brush group than the positive control group. This finding was supported by the SEM images (
Figure 2), which indicated that resin infiltration can be achieved successfully regardless of the thickness of the removed surface layers. Therefore, the present method minimizes the removal of surface layers consisting of high mineral contents. Also, in case of the post-orthodontic WSLs, applying 37% H
3PO
4 gel with the brush will not only enhance the esthetic appearance, but also prevent the progression of WSLs regardless of lesion activity.
In this study, by drawing comparisons between the positive control and the experimental group, we focused on the screening and technical practices involved in the suggested pretreatment before experimenting with natural lesions of human teeth. The bovine teeth were used as substitutes for human enamel, as reported in other studies.
26,
27 In our study, the artificial caries lesions were fabricated under identical conditions, and hence, their relatively uniform structure, lesion depths, and porous surfaces seem to be suitable for comparing the effects of different etching systems. Also, paired lesion halves derived from the same specimen were used for infiltration to minimize the gap among the specimens. However more experiments are required to confirm these findings and to apply these findings in natural lesions of human teeth because artificial and natural caries lesions and bovine and human teeth show different morphological structure, chemical, and physical properties.
28 Furthermore, the results obtained need to be verified by examining human teeth
in vivo to validate the possibility of using the application of phosphoric acid in the clinical approach.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download