Abstract
Purpose
This review aimed to evaluate the effectiveness of telemonitoring (TM) in the management of children and adolescents with asthma.
Methods
We searched Ovid-MEDLINE, Ovid-EMBASE, CENTRAL (Cochrane Central Register of Controlled Trials), CINAHL (Cumulative Index to Nursing and Allied Health Literature), and 5 domestic databases to identify randomized controlled trials (RCTs) published through December 2017. Two reviewers independently selected relevant studies, assessed methodological quality and extracted data. We performed a meta-analysis of TM versus usual care and summarized the intervention characteristics of included studies.
Results
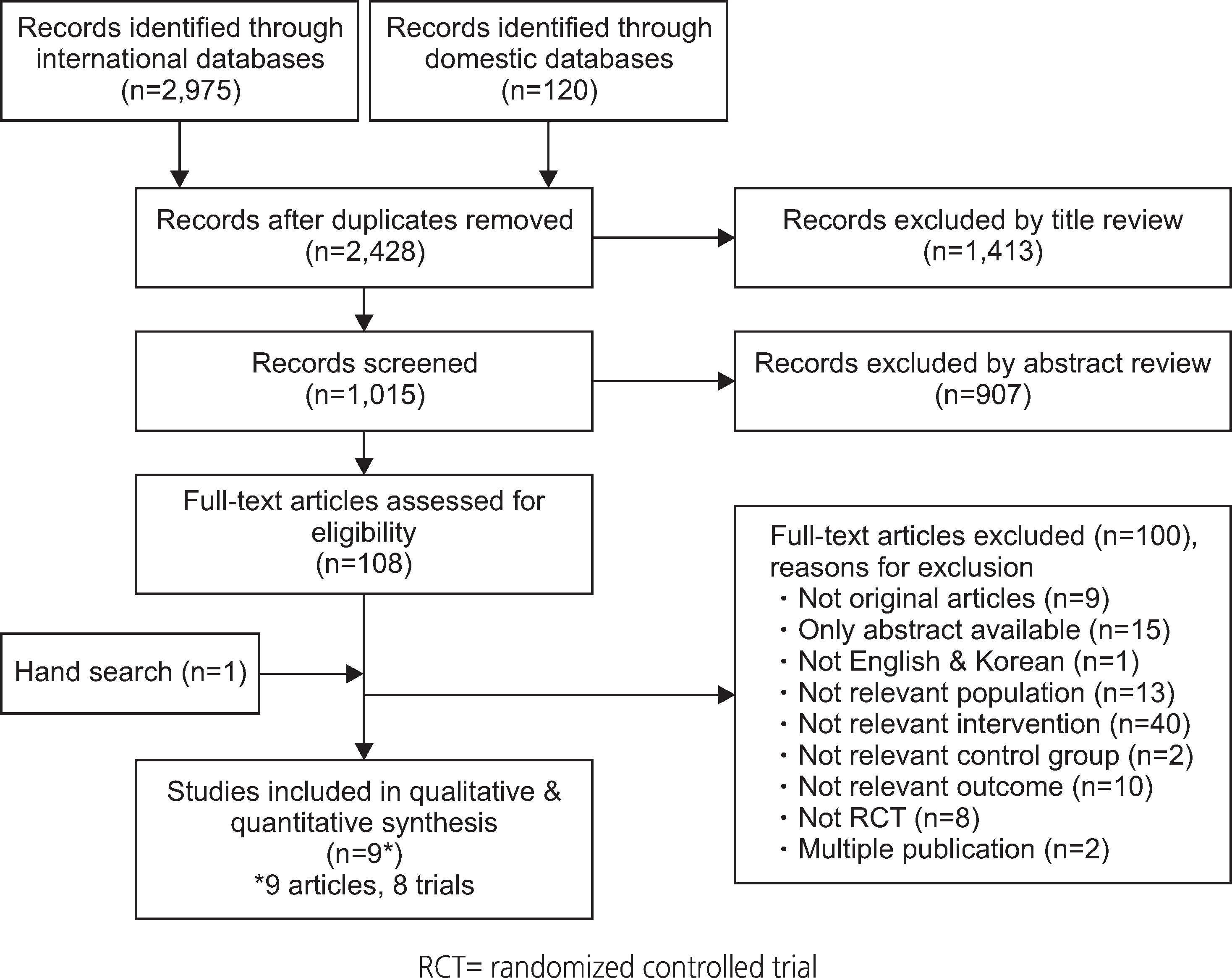
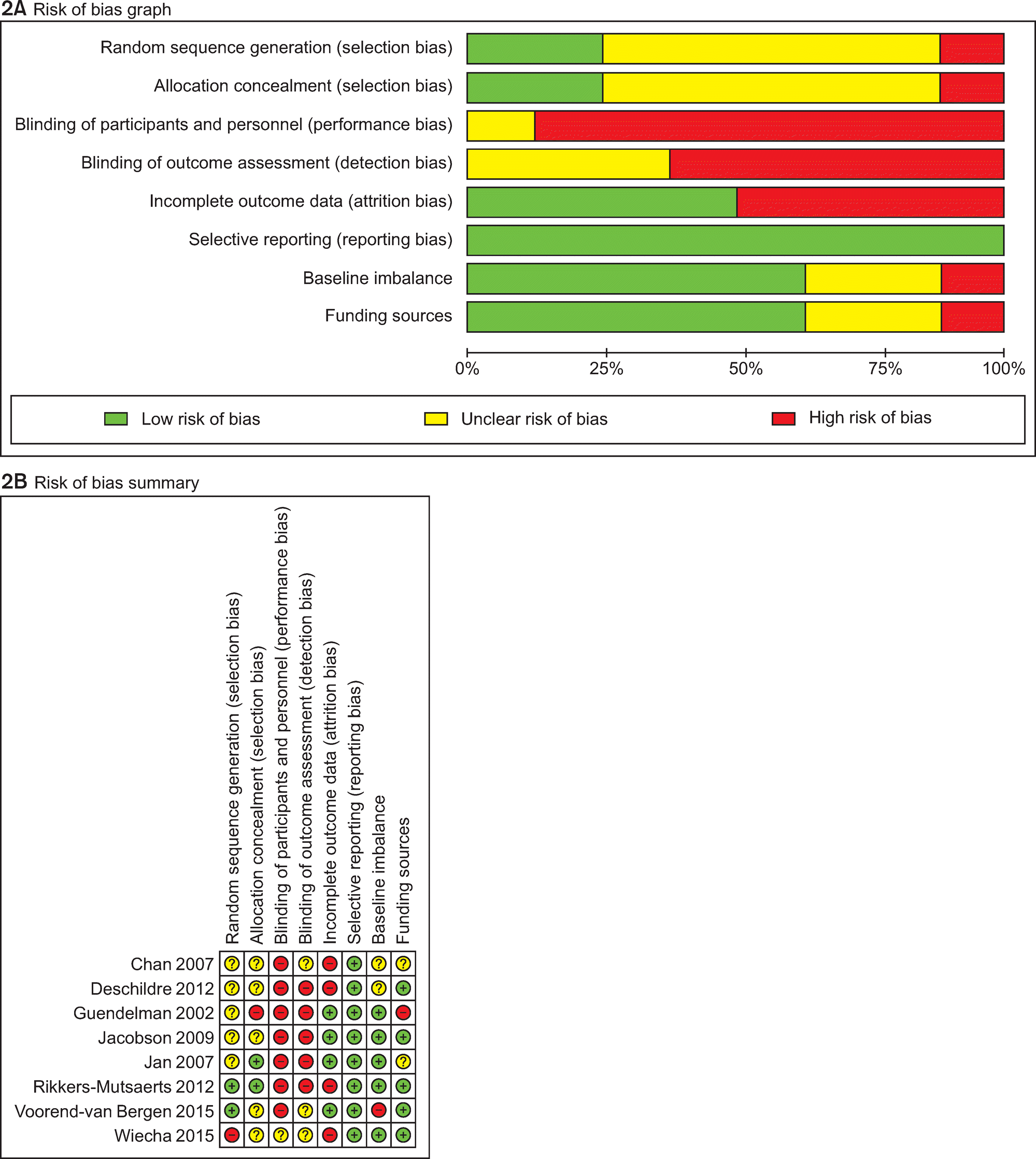
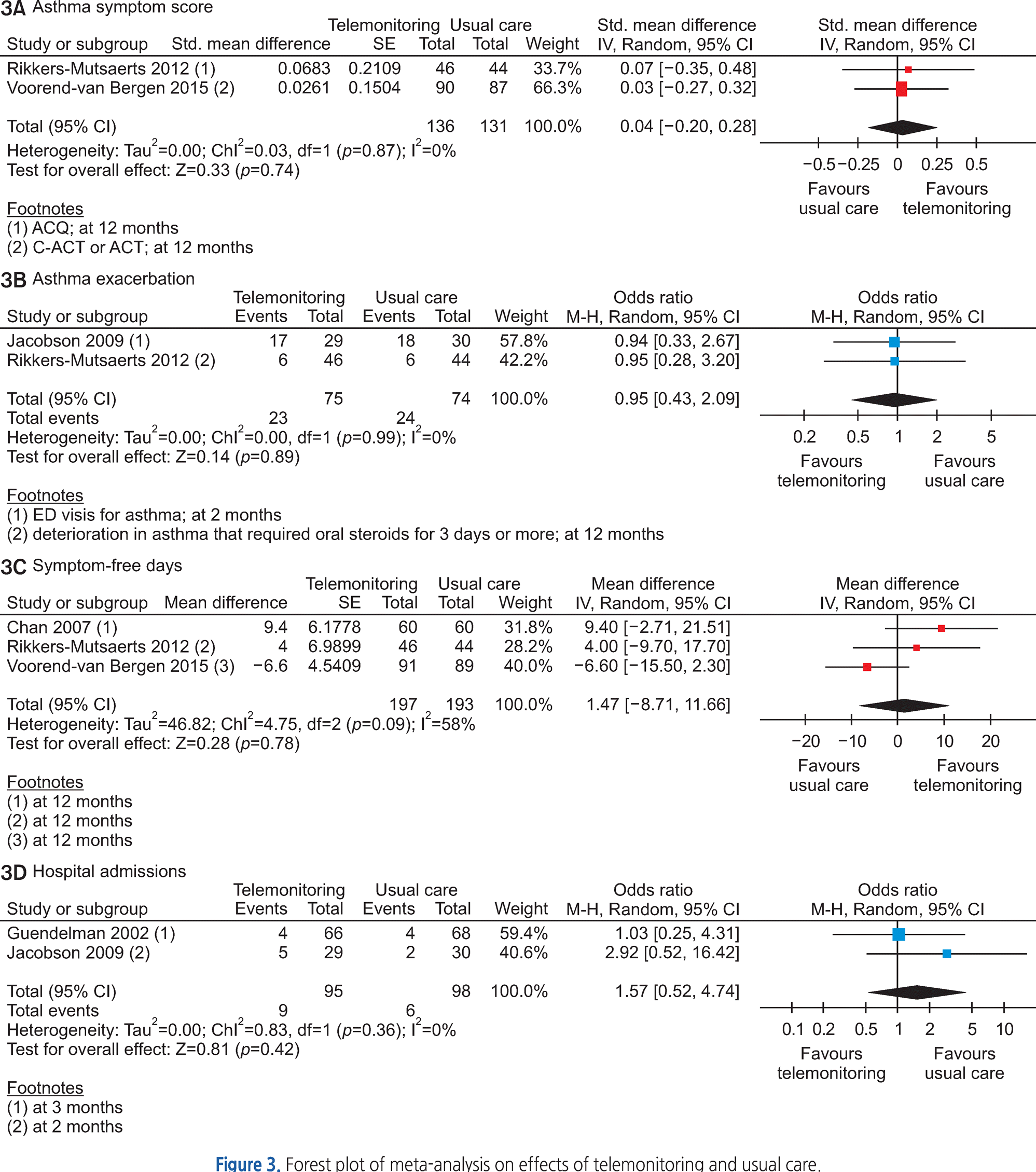
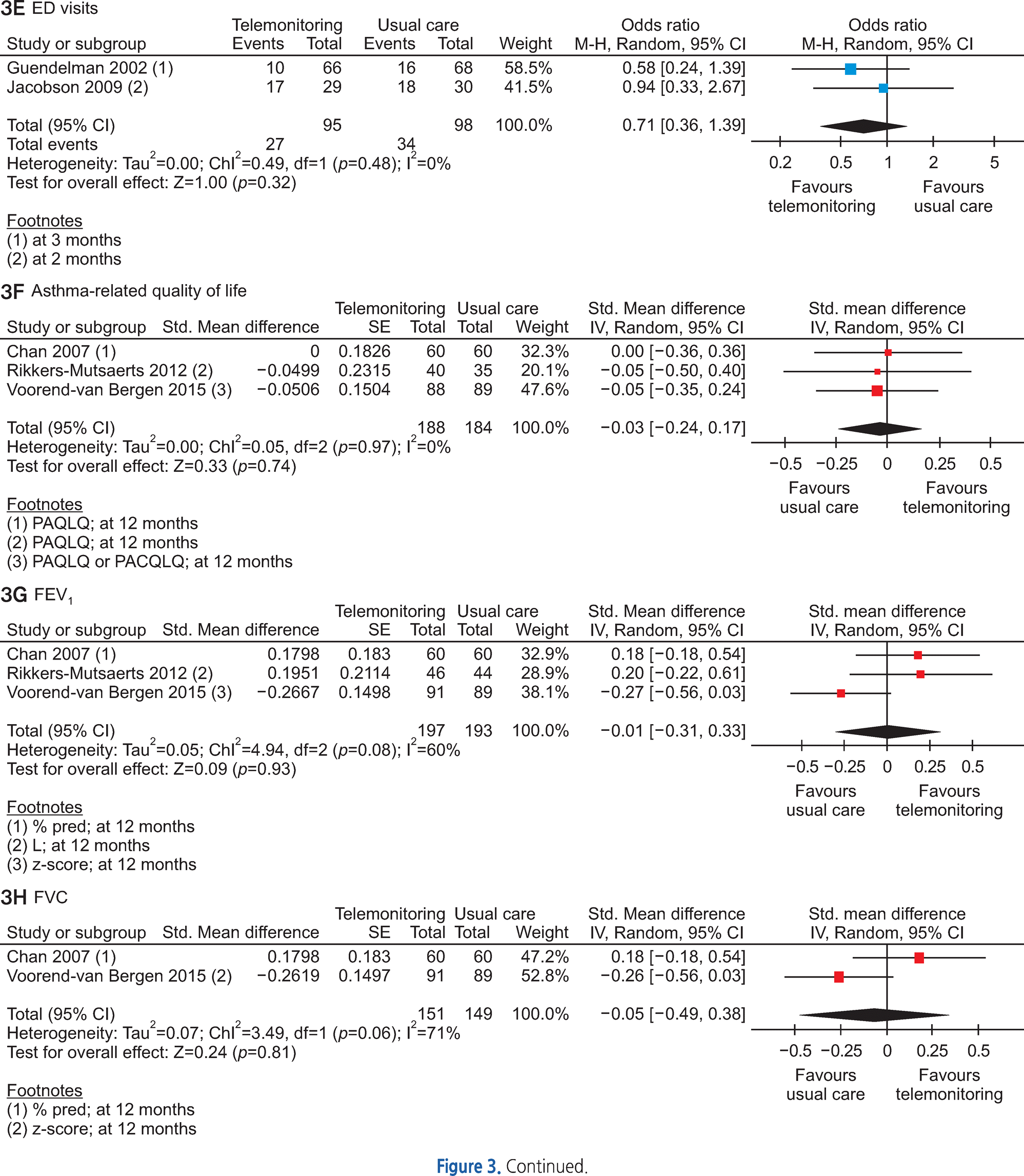
Of the 3,095 articles identified, 8 RCTs (9 articles) were included in this review. The type of TM intervention of included studies was varying across studies (transmitted data, transmission frequency, data review, etc.). The pooled asthma control score was not significantly different between TM and usual care (standardized mean difference 0.04, 95% confidence interval (CI) -0.20~0.28). Another pooled analysis demonstrated no statistically significant difference in asthma exacerbation between TM and usual care (odds ratio 0.95, 95% CI 0.43~2.09). Overall, the pooled results from these studies revealed that TM did not lead to clinically significant improvements in health outcomes, but some studies in our analysis suggested that TM increased patient medication adherence and intervention adherence.
Conclusion
The current evidence base does not demonstrate any differences between TM intervention and usual care, but TM intervention might be considered a promising strategy for the delivery of self-management support for children and adolescents with asthma. Further well-designed studies are needed to assess the effects on clinical outcomes.
References
1. Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention [Internet]. c2016. [cited 2016 Oct 12]. Available from:. https://ginasthma.org/wp-content/uploads/2016/04/wms-GINA-2016-main-report-final.pdf.
2. World Health Organization (WHO). World Health Organization Asthma Fact sheet No. 307 [Internet]. Geneva: WHO;c2017. [cited 2018 Jan 17]. Available from:. http://www.who.int/me-diacentre/factsheets/fs307/en/.
3. Fuchs O, Bahmer T, Rabe KF, von Mutius E. Asthma transition from childhood into adulthood. The Lancet Respiratory Medicine. 2017; 5(3):224–234. https://doi.org/10.1016/S2213-2600(16)30187-4.

4. Dean BB, Calimlim BC, Sacco P, Aguilar D, Maykut R, Tin-kelman D. Uncontrolled asthma: Assessing quality of life and productivity of children and their caregivers using a cross-sectional Internet-based survey. Health and Quality of Life Outcomes. 2010; 8:96. https://doi.org/10.1186/1477-7525-8-96.

5. Scottish Intercollegiate Guidelines Network (SIGN). British guideline on the management of asthma [Internet]. Edinburgh: SIGN;c2016. [cited 2018 Jan 25]. Available from:. http://www.sign.ac.uk/sign-153-british-guideline-on-the-management-of-asthma.html.
6. The Korean Academy of Asthma, Allergy and Clinical Immunology, The Korean Academy of Pediatric Allergy and Respiratory Disease, National Strategic Coordination Center for Clinical Research. Korean Guideline for Asthma [Internet]. [place unknown: publisher unknown];c2015. [cited 2016 Oct 12]. Available from:. http://www.allergy.or.kr/file/150527_01.pdf.
7. Pinnock H, Slack R, Pagliari C, Price D, Sheikh A. Understanding the potential role of mobile phone-based monitoring on asthma self-management: Qualitative study. Clinical and Experimental Allergy. 2007; 37(5):794–802. https://doi.org/10.1111/j.1365-2222.2007.02708.x.

8. Tuckson RV, Edmunds M, Hodgkins ML. Telehealth. New England Journal of Medicine. 2017; 377(16):1585–1592. https://doi.org/10.1056/NEJMsr1503323.

9. Flodgren G, Rachas A, Farmer AJ, Inzitari M, Shepperd S. Interactive telemedicine: Effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews. 2015; (9). CD002098. https://doi.org/10.1002/14651858.CD002098.pub2.

10. de Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V, Car J, Atun R. Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database of Systematic Reviews. 2012; 12:CD007459. https://doi.org/10.1002/14651858.CD007459.pub2.

11. Paré G, Moqadem K, Pineau G, St-Hilaire C. Clinical effects of home telemonitoring in the context of diabetes, asthma, heart failure and hypertension: A systematic review. Journal of Medical Internet Research. 2010; 12(2):e21. https://doi.org/10.2196/jmir.1357.

12. Zhao J, Zhai YK, Zhu WJ, Sun DX. Effectiveness of tele-medicine for controlling asthma symptoms: A systematic review and meta-analysis. Telemedicine Journal and e-Health. 2015; 21(6):484–492. https://doi.org/10.1089/tmj.2014.0119.

13. Marcano Belisario JS, Huckvale K, Greenfield G, Car J, Gunn LH. Smartphone and tablet self management apps for asthma. Cochrane Database of Systematic Reviews. 2013; (11). CD010013. https://doi.org/10.1002/14651858.CD010013.pub2.

14. McLean S, Chandler D, Nurmatov U, Liu J, Pagliari C, Car J, et al. Telehealthcare for asthma. Cochrane Database of Systematic Reviews. 2010; (10). CD007717. https://doi.org/10.1002/14651858.CD007717.pub2.

15. Kew KM, Cates CJ. Home telemonitoring and remote feedback between clinic visits for asthma. Cochrane Database of Systematic Reviews. 2016; (8). CD011714. https://doi.org/10.1002/14651858.CD011714.pub2.

16. Pijnenburg MW, Baraldi E, Brand PL, Carlsen KH, Eber E, Frischer T, et al. Monitoring asthma in children. European Respiratory Journal. 2015; 45(4):906–925. https://doi.org/10.1183/09031936.00088814.

17. Clift J. Connected asthma: How technology will transform care. Asthma UK Report. London: Asthma UK;2016.
18. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons;2011.
19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Journal of Clinical Epidemiology. 2009; 62(10):1006–1012. https://doi.org/10.1016/j.jclinepi.2009.06.005.

20. Kim SY, Park JE, Seo HJ, Lee YJ, Jang BH, Son HJ, et al. NECA’s guidance for undertaking systematic reviews and meta-analyses for intervention. Seoul: National Evidence-Based Healthcare Collaborating Agency;2011. p. 65–91.
21. Cochrane Collaboration. Review Manager (RevMan) [Computer Program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre;2014. Available from:. https://community.cochrane.org/help/tools-and-software/revman-5.
22. Guendelman S, Meade K, Benson M, Chen YQ, Samuels S. Improving asthma outcomes and self-management behaviors of inner-city children: A randomized trial of the Health Buddy interactive device and an asthma diary. Archives of Pediatrics and Adolescent Medicine. 2002; 156(2):114–120. https://doi.org/10.1001/archpedi.156.2.114.
23. Guendelman S, Meade K, Chen YQ, Benson M. Asthma control and hospitalizations among inner-city children: Results of a randomized trial. Telemedicine Journal and e-Health. 2004; 10(Suppl 2):6–14.

24. Deschildre A, Béghin L, Salleron J, Iliescu C, Thumerelle C, Santos C, et al. Home telemonitoring (forced expiratory volume in 1 s) in children with severe asthma does not reduce exacerbations. European Respiratory Journal. 2012; 39(2):290–296. https://doi.org/10.1183/09031936.00185310.

25. Rikkers‐Mutsaerts ERVM, Winters AE, Bakker MJ, van Stel HF, van der Meer V, de Jongste JC, et al. Internet‐based self ‐management compared with usual care in adolescents with asthma: A randomized controlled trial. Pediatric Pulmonology. 2012; 47(12):1170–1179. https://doi.org/10.1002/ppul.22575.
26. Voorend-van Bergen S, Vaessen-Verberne AA, Brackel HJ, Landstra AM, van den Berg NJ, Hop WC, et al. Monitoring strategies in children with asthma: A randomised controlled trial. Thorax. 2015; 70(6):543–550. https://doi.org/10.1136/thoraxjnl-2014-206161.

27. Jacobson JS, Lieblein A, Fierman AH, Fishkin ER, Hutchinson VE, Rodriguez L, et al. Randomized trial of an electronic asthma monitoring system among New York City children. American Journal of Managed Care. 2009; 15(11):809–814.
28. Chan DS, Callahan CW, Hatch-Pigott VB, Lawless A, Prof-fitt HL, Manning NE, et al. Internet-based home monitoring and education of children with asthma is comparable to ideal office-based care: Results of a 1-year asthma in-home monitoring trial. Pediatrics. 2007; 119(3):569–578. https://doi.org/10.1542/peds.2006-1884.

Table 1.
Characteristics of Included Studies
†272 participants were randomized to a telemonitoring group using ACT (Asthma Control Test) scores (n=91), to a control group (usual care) based on ACT without web feedback (n=89) and to a group for which FeNO (Fractional exhaled nitric oxide) and the ACT were used to monitor asthma (n=92). We chose not to include the FeNo group, as the comparison between telemonitoring and control groups was a purer comparison of the effect of telemonitoring than of use of FeNO.
2
Intervention Characteristics of Included Studies




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download