|
|
Exercise |
|
|
|
Mols et al., |
Population-based survey of |
Type: Physical Activity (walking, |
1) QOL (EORTC QLQ-C30) |
1) Physical activity was |
|
2015 |
colorectal cancer survivors: |
bicycling, gardening, |
2) Chemotherapy-induced |
associated with less CIPN- |
|
(Netherland) |
chemotherapy (n=506) vs. |
housekeeping, and sports |
peripheral neuropathy |
like symptoms and a higher |
|
no chemotherapy (n=1137) |
participation) |
(EORTC QLQ-CIPN20) |
QOL |
|
groups |
Format (setting): Individual (home) |
3) Physical Activity |
|
|
Chemotherapy: Yes |
Providers: Self |
Questionnaire (EPIC) |
|
|
Mean age (years): |
Session: <150 min/week or ≥150 |
|
|
|
Chemotherapy group |
min/week (Physical activity |
|
|
|
(66.7), no chemotherapy |
guideline) |
|
|
|
group (70.6) |
|
|
|
|
Schwenk |
|
Type: Interactive game-based |
1) CIPN -Severity (VPT score) |
1-3) No difference compared |
|
et al., 2016 |
RCT (pilot study) in older |
balance training |
− Pain (NRS) |
to baseline |
|
(USA) |
cancer patients undergoing |
Format (setting): Individual (cancer |
− Numbness in feet (NRS) |
4) Improved postural balance |
|
chemotherapy: CIPN |
center clinic) |
2) Health related QOL (SF- |
|
|
(n=22), experimental |
Providers: Supervisor (computer |
12) |
|
|
(n=11), and control (n=11) |
based training) |
3) Fear of falling (FES-I) |
|
|
groups |
Session: 45 min/session, |
4) Balance (Feet closed-EO, |
|
|
Chemotherapy: Yes |
2 times/week for 4 weeks |
EC, semi-tandem-EO) |
|
|
Mean age (years): 70±8.7 |
|
|
|
|
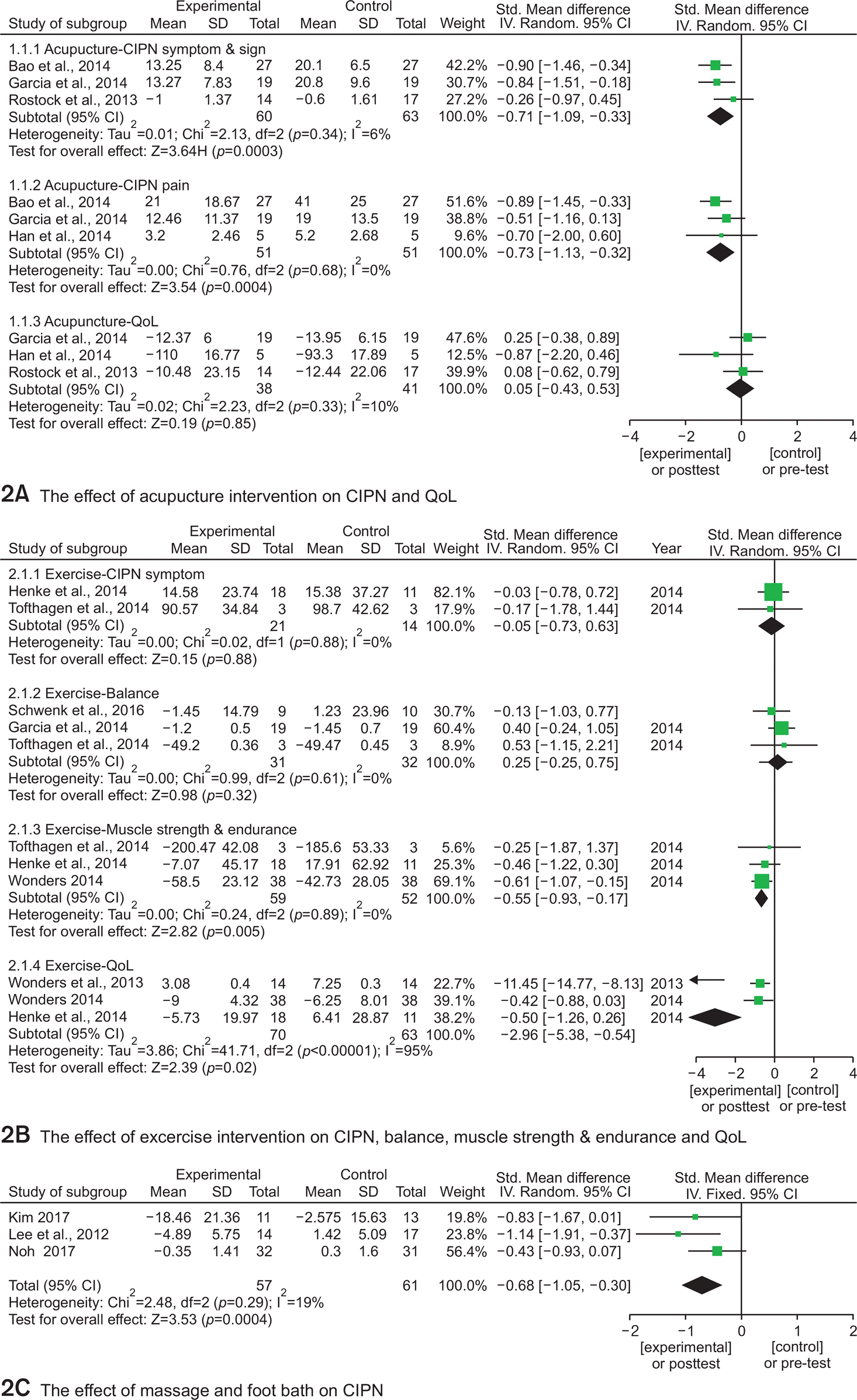
Henke et al., |
RCT in patients with |
Type: Strength and endurance |
1) ADL (Barthel Index) |
1) Increased ADL |
|
2014 (USA) |
stages Ⅲ-Ⅳ lung cancer |
training |
2) QOL (EORTC QLQ-C30) |
2) Increased QOL |
|
undergoing chemotherapy: |
Format (setting): Individual |
3) Peripheral neuropathy |
3) Improved peripheral |
|
CIPN (n=29), experimental |
(hospital) |
(EORTC QLQ -13) |
neuropathy |
|
(n=18) and control (n=11) |
Providers: Supervision of a licensed |
4) Functional capacity (6 |
4) Increased functional |
|
groups |
physiotherapist |
MWT, staircase walking) |
capacity |
|
Platinum-based |
Session: 1) Strength training with |
5) Muscle strength (curl-up |
5) Improved strength |
|
chemotherapy |
elastic band: |
test) |
|
|
Mean age: older than 18 |
3 sessions, 2-3 times/week during |
|
|
|
years |
3 cycles of chemotherapy |
|
|
|
|
2) Endurance training |
|
|
|
|
− Staircase walking: 2 min, |
|
|
|
|
5 times/week during 3 cycles |
|
|
|
|
of chemotherapy |
|
|
|
|
− 6-MWT: 6 min walking, |
|
|
|
|
5 times/week during 3 cycles |
|
|
|
|
of chemotherapy |
|
|
|
Tofthagen |
Single-group clinical trial in |
Type: Exercise |
1) Muscle strength |
1) Improved balance |
|
et al., 2014 |
colorectal cancer survivors |
Format (setting): Group |
(handheld isokinetic |
2) Improved strength |
|
(USA) |
undergoing chemotherapy: |
(community) |
dynamometer) |
3) Reduced neuropathic |
|
CIPN (n=3) |
Providers: Physical therapist |
2) Balance (postural |
symptoms |
|
Chemotherapy: Oxaliplatin |
Session: Strength training: |
instability, unipedal stance |
e |
|
Mean age (years): 69 |
20 min/session, 2 times/week |
time, TUG, mCTSIB, DGI) |
|
|
|
for 12 weeks |
3) Peripheral neuropathy |
|
|
|
stretching: 10~15 min/session, |
(CIPNAT, TNSr) |
|
|
|
2 times/week |
|
|
|
|
Balance training: 30 min/session, |
|
|
|
|
2 times/week |
|
|
|
Wonders, |
Single-group clinical trial in |
Type: Supervised individualized |
1) QOL (McGill) |
1) Improved QOL |
|
2014 (USA) |
cancer patients undergoing |
exercise program |
2) CIPN symptom (LANSS) |
2) Decreased Symptom of |
|
chemotherapy: CIPN (n=38) |
Format (setting): Individualized |
3) Fitness evaluation |
CIPN |
|
Chemotherapy: Taxanes and |
(medical center) |
– Flexibility (Lafayette |
3) Improved in VO2max, |
|
vinorelbine |
Providers: Certified cancer exercise |
Tester) |
muscular endurance and |
|
Mean age (years): male |
specialist |
− Muscular endurance/ |
flexibility |
|
(62.3±2.5), female (57.4± |
Session: 20~30 min/session, |
strength (curl up test) |
|
|
4.2) |
2 times/week for 12 weeks |
− Submaximal VO2max |
|
|
|
|
(Bruce treadmill test) |
|
|
Wonders |
Single-group clinical trial |
Type: Home-based exercise |
1) Step (Pedometer) |
1) Less of unpleasant skin |
|
et al., 2013 |
in breast cancer patients |
(resistance exercise & walking) |
2) QOL (McGill) |
sensations, abnormally |
|
(USA) |
undergoing chemotherapy |
Format (setting): Individual (home) |
3) Pain (Leeds Assessment |
sensitive to touch and |
|
(n=14). |
Providers: Kinesiologist |
of Neuropathic Symptoms |
sudden bursts (p=0.05). |
|
Chemotherapy: Taxanes and |
Session: walking: 10~30 min/ |
and Sign) |
2) Significantly improved of |
|
vinorelbine |
session, 2~5 times/wk for 10 wks |
|
QOL |
|
Mean age (years): 51.6±2.6 |
Resistance exercises: 3 times/week |
|
|
|
|
for 10 weeks. |
|
|
|
Streckmann |
RCT in lymphoma patients |
Type: Aerobic endurance and |
Primary: QOL (EORTC |
1) Improved QOL |
|
et al., 2014 |
undergoing chemotherapy: |
strength training, Sensorimotor |
QLQ-C30) |
2) Reduced symptoms of PNP- |
|
(USA) |
experimental group (n=30) |
training |
Secondary: |
related deep sensitivity |
|
and control group (n=31) |
Format (setting): Individual (medical |
1) Peripheral deep sensitivity |
3) Improved balance control |
|
Chemotherapy: Yes |
center) |
(tuning fork) |
on static and dynamic |
|
Mean age(years): Exp. (44), |
Providers: Certified or |
2) Activity level (MET) |
surface |
|
Cont. (48) |
physiotherapist |
3) Balance control (stable |
|
|
|
Session: 2 times/week for 36 weeks |
force plate/foam pad) |
|
|
|
1) Aerobic endurance training: |
4) Side-effects monitored: |
|
|
|
10~30 min walk on treadmill |
nutrition statue, anxiety |
|
|
|
2) Sensorimotor training: 20-s |
and depression (HADS) |
|
|
|
intervals, 1 min between exercise |
|
|
|
|
3) Strength training: 1 min at |
|
|
|
|
maximum force with hera-BandTM
|
|
|
|
|
Acupuncture |
|
|
|
Valentine- |
Retrospective case series |
Type: Acupuncture (Acupoints: |
CIPN grade (CTCAE) |
Improved the symptoms of |
|
Davis. 2015 |
of colorectal cancer |
individual basis) |
|
CIPN |
|
(USA) |
patients who underwent |
Format (setting): Individual |
|
|
|
chemotherapy with |
(hospital) |
|
|
|
gradeⅠ–ⅣCIPN (n=10) |
Providers: Unclear |
|
|
|
Chemotherapy: Oxaliplatin |
Session: 20 min, 2∼21 sessions |
|
|
|
Mean age (years): 53.1 |
|
|
|
|
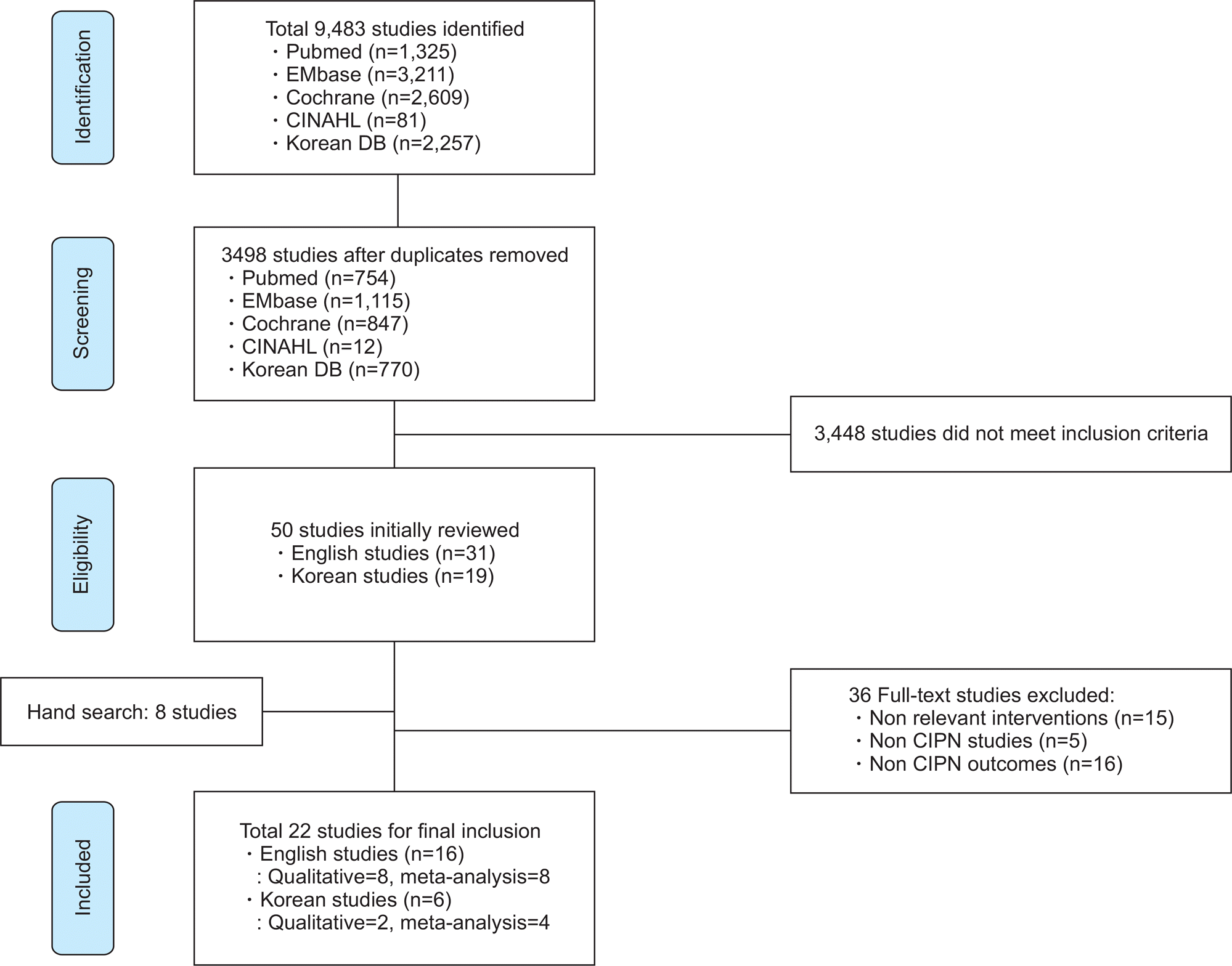
Bao et al., |
Single-arm pilot studyin |
Type: Acupuncture |
Signs and symptoms of |
Decreased pain and improved |
|
2014 (USA) |
multiple myeloma patients |
Format (setting): Individual |
neuropathy: |
function (walking, hand |
|
undergoing chemotherapy: |
(hospital) |
1) TNSc |
function: buttoning buttons, |
|
gradeⅡCIPN (n=27) |
Providers: Experienced |
2) FACT/GOG-NTx |
trouble feeling objects) |
|
Chemotherapy: Bortezomib |
acupuncturist |
3) NPS |
|
|
Mean age (years): 63 (49–77) |
Session: 20 min, 10 sessions for |
|
|
|
|
10 weeks |
|
|
|
Garcia et al., |
Single-arm pilot study in |
Type: Electroacupuncture |
1) Peripheral neuropathy |
1) Reductions in pain |
|
2014 (USA) |
) multiple myeloma patients |
Format (setting): Individual |
(FACT/GOG-Ntx) |
2) Improvements in FACT/ |
|
undergoing chemotherapy: |
(hospital) |
2) QOL (FACT-G survey) |
GOG/Ntx scores |
|
CIPN (n=19) |
Providers: Two licensed |
3) Pain (Brief Pain Inventory- |
3) Improvements in function |
|
Chemotherapy: Bortezomib |
acupuncturists with over 30 years’ |
Short Form) |
tests |
|
and thalidomide |
experience |
4) Function tests (coin test, |
|
|
Mean age (years): 64 |
Session: 20 min, 2~3 treatments/ |
button test, walking and |
|
|
|
week, 20 sessions for 9 weeks |
postural stability/fall risk) |
|
|
Han et al., |
Single-group clinical trial in |
Type: Acupuncture |
1) CIPN grade (CTCAE) and |
1) Reductions in CIPN pain |
|
2014 |
patients with myeloma or |
Format (setting): Individual |
pain (VAS) |
2) Improvements in QOL |
|
(Korea) |
lymphoma undergoing |
(hospital) |
2) QOL (FACT/GOG-Ntx) |
|
|
chemotherapy: gradeⅡCIPN |
Providers: Doctor of oriental |
3) CIPN (FACT/GOG-Ntx) |
|
|
(n=5) |
medicine |
|
|
|
Chemotherapy: Bortezomib |
Session: 15 min, 3 times/week |
|
|
|
and thalidomide |
during 3 weeks |
|
|
|
Mean age (years): 67.8±7.29 |
|
|
|
|
Lee et al., |
Case report of a patient |
Type: Acupuncture |
1) CIPN grade (CTCAE) |
1) Reductions in CIPN grade |
|
2014 |
with colorectal cancer |
Format (setting): Individual |
2) CIPN (EORTC QLQ- |
and pain |
|
(Korea) |
whounderwent |
(hospital) |
CIPN20) |
2) Improvements in QOL |
|
chemotherapy: CIPN (n=1) |
Providers: Doctor of Oriental |
2) QOL (EORTC QLQ-C30) |
|
|
Chemotherapy: Oxaliplatin |
medicine |
|
|
|
Mean age (years): 47 |
Session: 20 min, one time/week, |
|
|
|
|
15 sessions |
|
|
|
Park et al., |
Case report of a patient |
Type: Electroacupuncture |
1) CIPN grade (patient |
1) Reductions in CIPN pain |
|
2015 |
with breast cancer who |
Format (setting): Individual |
neurotoxicity |
2) Improvements in CIPN |
|
(Korea) |
underwent chemotherapy: |
(hospital) |
questionnaire) |
grade |
|
CIPN (n=1) |
Providers: Doctor of Oriental |
2) Pain (VAS) |
|
|
Chemotherapy: Docetaxel |
medicine |
|
|
|
Age(years): 56 |
Session: 20 min, 2 times/day during |
|
|
|
|
2 weeks |
|
|
|
Ogawa |
Single-arm pilot studyin |
Type: Acupuncture (contact needle |
1) CIPN symptoms (CTCAE), |
1) Improved the symptoms of |
|
et al., 2013 |
patients with colorectal and |
therapy: Traditional Japanese |
FACT/GOG-Ntx |
CIPN |
|
(Japan) |
breast cancer undergoing |
methods of acupuncture) |
|
|
|
chemotherapy (n=4) |
Format (setting): Individual |
|
|
|
and who had undergone |
(hospital) |
|
|
|
chemotherapy (n=2) with |
Providers: Senior acupuncturist |
|
|
|
grade I–II CIPN |
Session: 30~60 sec/session, 4~6 |
|
|
|
Chemotherapy: Taxanes and |
treatments for 3 months |
|
|
|
oxaliplatin |
|
|
|
|
Mean age (years): 64.3 |
|
|
|
|
Rostock |
Four-arm RCT in |
Type: Electroacupuncture (EA) or |
1) CIPN complaints (Detailed |
1) No improvement inthe |
|
et al., 2013 |
cancer patientswith |
Hydroelectric bath (HB) or vitamin |
questionnaires) |
symptoms of CIPN |
|
(Germany) |
CIPNundergoing: |
B1 and B6 (vitamin B) vs. placebo |
2) Neuropathic symptoms |
2) No improvement in QOL |
|
experimental group (n=42) |
Format (setting): Individual |
(NRS, Neuropathy score, |
|
|
and control group (n=17) |
(hospital) |
Electroneurographical |
|
|
Chemotherapy: Taxanes and |
Providers: Two trained physicians |
test) |
|
|
platinum derivatives |
Session: 15 min, 8±1 sessions for 3 |
3) QOL (EORTC QLQ-C30) |
|
|
Mean age (years): Exp. (EA: |
weeks |
|
|
|
49.9±9.6, Con. (52.0±8.1) |
|
|
|
|
Donald et al., |
Retrospective service |
Type: Acupuncture |
1) CIPN symptom (evaluation |
n 1) Improvement in PN |
|
2011 (UK) |
evaluation of cancer |
Format (setting): Individual |
form) |
symptoms |
|
patients who underwent |
(outpatient clinic) |
2) Additional acupuncture |
2) Reduction in analgesic use |
|
chemotherapy: CIPN (n=18) |
) Providers: Qualified nurses |
benefits |
and improved sleeping |
|
Chemotherapy: Bortezomib, |
Session: 30~45 min/session, 6 |
|
patterns. |
|
thalidomide, taxanes and |
treatments for 6weeks |
|
|
|
platinum derivatives |
|
|
|
|
Mean age (years): |
|
|
|
|
51.83±12.97 |
|
|
|
|
Schroeder |
NRCT (pilot study) in |
Type: Acupuncture (traditional |
1) Nerve conduction velocity |
1) Improved the symptoms of |
|
et al., 2011 |
cancer patients with CIPN |
Chinese medicine) |
(nerve conduction study) |
PN |
|
(Germany) |
undergoing chemotherapy: |
Format (setting): Individual |
|
|
|
experimental group (n=6) |
(hospital) |
|
|
|
and control group (n=5) |
Providers: Senior physician who had |
|
|
|
Chemotherapy: Taxanes and |
received >1000 h of acupuncture |
|
|
|
platinum derivatives |
training |
|
|
|
Mean age (years):Exp. (64), |
Session: 20 min/session, 10 |
|
|
|
Con. (65) |
treatments for 3months |
|
|
|
Wong & |
Case series of gynecological |
Type: Acupuncture |
1) Pain |
1) Improvements in sensation |
|
Sagar, 2006 |
cancer patients who |
Format (setting): Individual |
2) Physical assessment |
gait, and balance |
|
(Canada) |
underwent with gradeⅡ– |
(hospital) |
(sensation, gait, and |
2) Decreased analgesic dosage |
|
ⅢCIPN (n=5) |
Providers: Certified therapist in |
balance) |
and symptoms (pain, |
|
Chemotherapy: Carboplatin |
medical acupuncture |
|
numbness and tingling) |
|
(total, 3500 mg) and |
Session: First: 30~45 min/session, |
|
|
|
paclitaxel (total, 1860 mg) |
once a week for 12 weeks |
|
|
|
Age: 60~71 years |
|
|
|
|
|
Massage / Foot bath |
|
|
|
Noh 2017 |
RCT in patients with |
Type: Self-foot reflexology |
1) CIPN symptoms and |
1) Decreased CIPN symptoms |
|
(Korea) |
gynecologic cancer |
Format (setting): Individual |
interference (CIPNAT) |
andinterference |
|
undergoing chemotherapy: |
(hospital andhome) |
2) Peripheral skin |
2) Increased peripheral skin |
|
experimental group (n=32) |
Providers: Nurse (certified foot |
temperature |
temperature |
|
and control group (n=31) |
reflexology) |
3) Anxiety and depression |
3) Reduced anxiety and |
|
Chemotherapy: Taxane |
Session: 20 min/session, 3 times/ |
(HADS) |
depression |
|
andplatinum |
week for 6 weeks |
|
|
|
Mean age (years): Exp. |
|
|
|
|
(56.34±9.04), Con. |
|
|
|
|
(55.36±9.96) |
|
|
|
|
Lee et al., |
NRCT in patients with |
Type: Foot reflexology |
1) CIPN (EORTC QLQ- |
1) Less peripheral neuropathy |
|
2012 |
various cancer types |
Format (setting): Individual |
CIPN20) |
and symptom distress |
|
(Korea) |
undergoing chemotherapy: |
(hospital and home) |
2) Pain (VAS) |
2) No difference in anxiety |
|
experimental group (n=14) |
Providers: Nurse (certified foot |
3) Symptom distress (Distress |
s and depression |
|
and control group (n=17) |
reflexology) |
Thermometer) |
|
|
Chemotherapy: Oxaliplatin |
Session: 20 min/session, 5 times/ |
4) Anxiety and depression |
|
|
Mean age (years): Exp. (59.9± |
± week for 4 weeks |
(HADS) |
|
|
8.12), Con. (57.7± 8.88) |
|
|
|
|
Cunningham |
Case report ofesophageal |
Type: Manual therapy (therapeutic |
1) MD Anderson Symptom |
1) CIPN symptoms reduced to |
|
et al., 2011 |
patient at stage III who |
massage) |
Inventory |
grade I |
|
(USA) |
underwent chemotherapy |
Format (setting): Individual |
2) Micromuscular blood flow |
2) Increased temperature in |
|
with grade ⅡCIPN (n=1) |
(massage therapy room) |
(Infrared thermometry) |
each extremity |
|
Chemotherapy: Docetaxel |
Providers: Two licensed massage |
|
|
|
and cisplatin |
therapist |
|
|
|
Age: 45 years |
Session: 25 min/session, 3 times |
|
|
|
|
/week for 6 weeks |
|
|
|
Kim, 2017 |
NRCT of patients with |
Type: Foot bath therapy |
1) CIPN symptom and pain |
1) CIPN symptom and pain |
|
(Korea) |
various cancer types who |
Format (setting): Individual |
2) CIPN interference |
partially supported |
|
underwent chemotherapy |
(hospital) |
|
2) CIPN interference partially |
|
with experimental (n=11) |
Providers: Nurse |
|
supported |
|
and control (n=13) groups |
Session: 25 min/session, 4 times |
|
|
|
Chemotherapy: Yes |
/week for 2 weeks, 11 sessions |
|
|
|
Mean age (years): Exp. |
|
|
|
|
(44.55±10.25), Con. |
|
|
|
|
(49.77±8.14) |
|
|
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download