Dear Editor,
Mitochondrial alanyl-tRNA synthetase 2 (AARS2) plays a role in the initiation of mitochondrial translation by charging tRNAs with their appropriate amino acids. Various mutations of the AARS2 gene (Mendelian Inheritance in Man 612035) have been found in severe infantile cardiomyopathy and in 15 adults with progressive diffuse leukoencephalopathy that is systematically associated with ovarian failure in females (8 patients).123 A recently reported genetic screening study involving undiagnosed patients with adult-onset leukoencephalopathy suggested that AARS2-related leukoencephalopathy could account for a substantial subset of those patients, highlighting the need for a deeper phenotyping of the disease.4 Here we report a 41-year-old woman who suffered from a 6-year-long history of isolated severe cognitive and behavioral frontal syndrome associated with bilateral frontal cystic leukoencephalopathy, ovarian failure, and a novel AARS2 genotype.
The patient was born healthy from nonconsanguineous parents, with no family history of neurological or psychiatric disorders. She underwent a left ovariectomy and a right ovarian cystectomy in adolescence due to mucinous cysts. She was subsequently diagnosed with ovarian failure thought to be caused by the ovarian surgeries. She developed normally, working as a secretary, but lost her job at 35-years-old due to the onset of dysexecutive symptoms, disinhibition (hyperorality, excessive spending, and impulsivity), and spatial disorientation. She presented at our unit at the age of 36-years with a severe behavioral and cognitive frontal syndrome, with a Mini Mental State Examination score of 29/30, a Frontal Assessment Battery score of 6/18, altered recalling with storage sparing in episodic memory, altered inhibition processes on the Trail-Making Test and Stroop Test, and altered social cognition on the Ekman Faces Test and Faux Pas Recognition Test; other findings of neurological examination were normal. The patient's frontal behavior worsened slowly up to the age of 41 years, leading her to steal sweaters in shops or run naked in the street while yelling. The patient is still alive, with her parents helping her to perform daily activities.
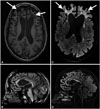
Brain magnetic resonance imaging (MRI) revealed frontal white-matter abnormalities that were stable over a 5-year period (Fig. 1). Basic cerebrospinal fluid (CSF) analysis produced normal findings, with no meningitis, elevated CSF protein, or oligoclonal bands detected. Other laboratory tests did not support 1) infectious diseases (negative for HIV 1–2, Cytomegalovirus, epstein-barr virus, syphilis antibodies, varicella-zona virus, and Lyme in serum, and for Lyme, toxoplasma, and herpes simplex virus 1–2 PCR in CSF), 2) autoimmune diseases (conversion enzyme was normal along with the usual immunological laboratory findings in plasma; negative for intracellular and cell-surface neuronal autoantibodies in both serum and CSF), or 3) metabolic diseases (normal folate, lactate, amino-acid chromatography, total homocysteine, arylsulfatase, galactocerebrosidase, cholestanol, oxysterols, and very-long-chain fatty acids in serum; normal organic acids in urine; and normal folate and lactate in CSF). Electroencephalogram, electromyogram, and muscle-biopsy findings were normal.
Written informed consents for genetic testing were obtained from the patient and her parents. DNA was extracted from peripheral blood samples, and the application of targeted nextgeneration sequencing of a panel of 131 genes (Supplementary Table 1 in the online-only Data Supplement) implicated in common leukoencephalopathies and spastic paraplegias was performed following the manufacturer's protocol (SureSelectQXT Target enrichment kit, Agilent®; Santa Clara, CA, USA on a NextSeq500 sequencer, Illumina®; San Diego, CA, USA). We found two mutations in AARS2 (NM_020745.3) at the compound heterozygous status: c.595C>T/p. (Arg199Cys) and c.647dupG/p. (Cys218 Leufs*6) (Supplementary Fig. 1 in the online-only Data Supplement). Genetic analysis of her parents confirmed biallelic segregation consistent with autosomal recessive inheritance. The c.595C>T mutation has only been reported for three other patients with leukoencephalopathy,24 while the c.647dupG mutation has only been detected in one child with multiple mitochondrial respiratory chain complex deficiency associated with a cardiomyopathy.5 The duplication of guanine in position c.647 induces the apparition of a premature stop codon downstream of the 218 cysteine residue in exon 4 that encodes for part of the alanyl-tRNA synthetase core domain.
To the best of our knowledge, no such genotype with these two combined mutations has been previously reported. The phenotype is also consistent with the predictions of Euro et al.6 in 2015 that one nonsense mutation together with the milder p.Arg199Cys mutation induces a leukodystrophic phenotype. The underlying mechanism proposed by Euro et al.6 is a reduced rate of tRNA aminoacylation in the synthetase induced by changes in adenosine triphosphate binding and impaired alanyl-adenylate formation.
Twelve of the 15 patients reported with AARS2-related leukoencephalopathy experienced cognitive decline and behavioral changes, together with other neurological deficits such as ataxia and upper motor neuron signs. All of the reported patients showed a diffuse leukoencephalopathy and a severe worsening of their clinical state over the course of a few years, until bed confinement or death. The clinical and radiological phenotype in our patient was unique in many ways. Exclusive cognitive and behavioral changes (with specific involvement of frontal-lobe functions and relative sparing of memory and instrumental functions) were found at presentation, without any other neurological deficits, including at the last follow-up. This is consistent with the MRI findings showing a selective frontal leukoencephalopathy. Moreover, the symptoms of our patient worsened very slowly, and she is still alive today.
In conclusion, we have described a new AARS2-related clinicoradiological phenotype that should prompt AARS2 sequencing early in the diagnostic process.
Figures and Tables
Fig. 1
Brain magnetic resonance imaging changes in our patient. A: Axial T1-weighted sequence. B: Sagittal T1-weighted sequence. C: Axial FLAIR T2-weighted sequence. D: Sagittal FLAIR T2-weighted sequence. Confluent white-matter abnormalities are restricted to the frontal lobes with high T2-weighted FLAIR signals (arrows in C) and low T1-weighted signals, centered by a very low T1-weighted signal, corresponding to a cyst (arrows in A). Frontal lobes are atrophic, with enlargement of the contiguous part of the lateral ventricles. The corpus callosum genu is highly atrophic with a high T2-weighted FLAIR signal. Mild atrophy of the cerebellum is also noted. FLAIR: fluid-attenuated inversion recovery.
References
1. Götz A, Tyynismaa H, Euro L, Ellonen P, Hyötyläinen T, Ojala T, et al. Exome sequencing identifies mitochondrial alanyl-tRNA synthetase mutations in infantile mitochondrial cardiomyopathy. Am J Hum Genet. 2011; 88:635–642.

2. Szpisjak L, Zsindely N, Engelhardt JI, Vecsei L, Kovacs GG, Klivenyi P. Novel AARS2 gene mutation producing leukodystrophy: a case report. J Hum Genet. 2017; 62:329–333.

3. Dallabona C, Diodato D, Kevelam SH, Haack TB, Wong LJ, Salomons GS, et al. Novel (ovario) leukodystrophy related to AARS2 mutations. Neurology. 2014; 82:2063–2071.

4. Lynch DS, Zhang WJ, Lakshmanan R, Kinsella JA, Uzun GA, Karbay M, et al. Analysis of mutations in AARS2 in a series of CSF1R-negative patients with adult-onset leukoencephalopathy with axonal spheroids and pigmented glia. JAMA Neurol. 2016; 73:1433–1439.

Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2018.14.3.420.
Supplementary Table 1
Description of the 131 genes implicated in common leukoencephalopathies, and spastic paraplegias, and tested by the targeted next-generation sequencing panel used in this study
Supplementary Fig. 1
Schematic representation of the variants identified in an next-generation sequencing analysis using the Alamut® (Interactive Biosoftware, Rouen, France) visual tool. The reference sequence (NM) and Binary Alignment Map alignment of the proband are shown. Boxes list the counts for the total and absolute reads and the allele frequency (%) of the two mutations.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download