To Editor,
Uterine carcinosarcoma (UCS) is a high-grade endometrial cancer [
1]. Although UCS is a rare tumor its proportion among endometrial cancers has been gradually increasing over recent decades [
2]. UCS is a biphasic tumor with the sarcomatous element arising as a result of dedifferentiation from the carcinoma component via epithelial-mesenchymal transition [
3]. UCS exhibits aggressive tumor behavior and the associated prognosis is generally poor even in early-stage disease [
45]. Therefore, it is imperative that we identify predictors for survival to aid in the management of UCS.
Cancer antigen 125 (CA-125) is a transmembrane mucin protein encoded by the
MUC16 gene [
6]. CA-125 has been widely used as a diagnostic or prognostic marker in gynecologic malignancies including epithelial ovarian cancer and endometrial cancer [
7]. In UCS, there is little evidence examining the role of CA-125 as a prognostic indicator for survival. A prior study used a cutoff value of 30 IU/L (normal <30 IU/L vs. abnormal ≥30 IU/L), demonstrating its utility for the predicting advanced-stage disease and decreased survival [
8]. However, this cutoff was arbitrarily chosen without rationale provided for how this cutoff value was set. Harano et al. [
9] reported CA-125 to be a prognostic factor but the details regarding the cutoff value were not provided. Another study found no impact on survival but was limited by sample size [
10].
The rarity of UCS as a disease entity has led to both the understudy of the disease itself and of the role of prognostic markers like CA-125 in its management. The objective of this study was to examine the association of CA-125 and survival in UCS by introducing a clinically useful CA-125 cutoff to predict a subgroup of patients with a considerably higher risk of recurrence and progression.
This is an ancillary analysis of a previously organized large-scale multicenter retrospective study of UCS (n=906) [
4]. This dataset consisted of consecutive cases of women with stage I–IV UCS who underwent primary hysterectomy-based surgical treatment between 1993 and 2013. Among the dataset, 615 women with available pretreatment CA-125 levels were eligible for the current study (median=23; interquartile range [IQR]=47). Among available cases, patient demographics, tumor characteristics, treatment type, and survival were abstracted. Institutional Review Board approval was obtained from each participating site.
Patient demographics included age, race/ethnicity, pretreatment serum CA-125 level, and body mass index. Tumor characteristics included carcinoma type, sarcoma type, sarcoma dominance, lymphovascular space invasion (LVSI), depth of myometrial invasion, lymph node status (pelvic and/or para-aortic), and cancer stage. Treatment type included surgical performance with residual disease at surgery, adjuvant therapy (chemotherapy and radiotherapy). Survival outcomes included progression-free survival (PFS), defined as the time interval between surgery and the first recurrence/progression of disease or death due to UCS. Patients who were alive at the last follow-up were censored.
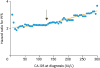
A CA-125 cutoff value was examined every 5 IU/L increment change between 5 and 300 IU/L. At each cutoff, a Cox proportional hazard regression test was used to determine the unadjusted hazard ratio (HR) and 95% confidence interval (CI) for PFS. Temporal trend analysis was performed to identify the reflection point for HR. The Joinpoint Regression Program (version 4.6.0.0) provided by the National Cancer Institute was utilized for evaluating temporal trends and reflection points for HR changes. The presence of temporal trend was examined with a linear segmented regression test, and log-transformation was performed to determine the annual percent change (APC) and 95% CI.
Based on this exploratory analysis, we found that the significance of HR for PFS significantly increases at the CA-125 level of 125 IU/L: APC 0.09 vs. 0.21 for CA-125 5–125 IU/L vs. 125–300 IU/L (both, p<0.001;
Fig. 1). We therefore defined the CA-125 levels together with the prior study cutoff as follows: normal <30 IU/L, abnormal 30-124 IU/L, and marked high-risk for recurrence ≥125 IU/L. The CA-125 cutoffs were then correlated to patient demographics, tumor characteristics, and survival.
To examine the independent association of the CA-125 level and PFS, a Cox proportional hazard regression model with conditional backward method was used for multivariate analysis. The covariates entered in the initial model included were based on a priori survival factors as demonstrated in a previous study: age, country, residual disease, carcinoma type, sarcoma type, sarcoma dominance, tumor size, depth of myometrial invasion, LVSI, cancer stage, chemotherapy, and radiotherapy [
4]. The least significant covariates were removed from the model until the final model included the covariates with p<0.05 level.
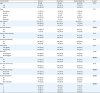
There were 615 cases with available pretreatment CA-125 results. Patient demographics and tumor characteristics based on the CA-125 classification are shown in
Table 1. The majority of the study population had normal CA-125 (n=355, 57.9%) followed by abnormal CA-125 (n=167, 27.2%), and marked high-risk CA-125 (n=91, 14.8%).
CA-125 levels were significantly correlated with surgical performance, LVSI, myometrial tumor invasion, lymph node status, and cancer stage (all, p<0.05). Specifically, tumors within the marked high-risk CA-125 group were more likely to have LVSI, deep myometrial invasion, more lymph node metastasis, and higher cancer stage compared to other groups. Additionally, among the 268 cases of stage III–IV disease, CA-125 levels were significantly associated with residual disease at surgery: normal 13.5%, abnormal 25.0%, and marked high-risk 44.9% (p<0.001).
The median follow-up time for the censored cases was 47.8 months (IQR=64.8). There were 289 cases that experienced a recurrence or progression, and 205 cases resulting in death from UCS during the follow-up period. On univariate analysis, CA-125 level (by group) was significantly correlated with 5-year PFS rates: normal 60.2%, abnormal 36.1%, and marked high-risk 23.3% (p<0.001,
Fig. 2). The median PFS was not reached for the normal group but was 21.1 months for the abnormal group and 10.1 months for the marked high-risk group (overall, 40.5 months). The marked high-risk CA-125 level was also associated with significantly decreased PFS when compared to the abnormal CA-125 level (HR=1.58; 95% CI=1.15–2.18; p=0.005).
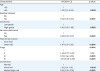
On multivariate analysis, the CA-125 classification remained an independent prognostic factor for PFS (
Table 2). The abnormal CA-125 level was associated with a 35% increased risk for recurrence or progression compared to the normal CA-125 level (adjusted-HR=1.35; 95% CI=1.01–1.81; p=0.042); and the marked high-risk CA-125 level was associated with 89% increased risk (adjusted-HR=1.89; 95% CI=1.30–2.74; p<0.001). Old age, residual disease at surgery, postoperative chemotherapy use, carcinoma type, sarcoma dominance, deep myometrial invasion, and cancer stage remained independent prognostic factors for PFS in this study population (all, p<0.05). Marked high-risk CA-125 level had the third largest magnitude of statistical significance for PFS following stage III–IV disease (adjusted-HR=2.19–3.21) and residual disease (adjusted-HR=1.96).
This study found that the risk-based CA-125 classification introduced via a logistic approach in our analysis is useful for identifying a subgroup of women with a substantially increased risk of tumor recurrence and progression in UCS. This novel classification adds new insights to a previously proposed CA-125 cutoff; specifically, a CA-125 cutoff of 125 IU/L is associated with nearly 50% increased risk of recurrence/progression compared to lower level of CA125 (30–124 IU/L). This is clinically meaningful in the management of women with UCS.
Although a higher CA-125 level is associated with aggressive tumor features such as deep myometrial invasion, LVSI, nodal metastasis, and advanced-stage disease (
Table 1), we found that a marked high-risk CA-125 level was independently associated with decreased PFS after controlling for these tumor factors. This possibly implies that a high level of CA-125 is likely derived from UCS tumors with increased MUC16 gene activity. The MUC family could potentially be a target for cancer vaccine therapy [
11]. It would be of particular interest to look at the therapeutic implications of an anti-MUC vaccine in marked high-risk UCS cases.
Our study found that the marked high-risk CA-125 group had a considerably increased risk of residual disease at surgery in advanced-stage disease (44.9%). Although our study does not have information regarding an objective assessment of tumor burden via preoperative imaging, this information on CA-125 can be used to help surgeons guide preoperative assessment of women with UCS. If surgeons know that the risk of residual disease is highly likely based on the pretreatment CA-125 level and imaging, unnecessary procedures, aggressive intervention, and futile laparotomy may be avoidable in order to reduce surgical morbidity. It is paramount to note that residual disease at surgery is an independent prognostic factor associated with decreased PFS. The role of neoadjuvant chemotherapy for unresectable UCS has not been examined and warrants future investigation [
12].
Clinicians need to be aware that the prevalence of marked high level of CA-125 in UCS is not rare, with approximately one in seven women with UCS having a marked high-risk level CA-125. In such a population, with a substantially increased risk of tumor recurrence or progression, close surveillance with CA-125 may be of value despite evidence that routine CA-125 in women with endometrial cancer is currently not supported by the Society of Gynecologic Oncology [
13]. Whether maintenance therapy after the adjuvant therapy in this high-risk group reduces the risk of recurrence is of interest and merits further study.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download