INTRODUCTION
MATERIALS AND METHODS
1. Cell culture
2. Three-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays
3. Apoptosis assessment
4. Dual luciferase reporter assays
5. TOPflash-luc activity assays
6. Chromatin immunoprecipitation (ChIP) assays
7. Measurement of cytochrome C release from mitochondria
8. Quantitative reverse transcription polymerase chain reaction (PCR)
9. Western blotting analysis
10. Statistical analysis
RESULTS
1. PAB negatively affects the survival of cervical cancer cells in a time- and concentration-dependent manner
 | Fig. 1PAB inhibits the survival of cervical cancer cells in a time- and concentration-dependent manner. Five different cervical cancer cell lines (HeLa, SiHa, Caski, C33A, and MS751) were treated with different concentrations of PAB (0, 2.5, 5.0, 10, 20, 40 μM) for 24, 48, and 72 hours. The MTT assay was used to measure the inhibitory effect of PAB on cell proliferation (n=3). Data are expressed as mean values ± standard deviation for 3 independent experiments.MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PAB, pseudolaric acid B.
*p<0.05 was considered significant.
|
2. PAB increases the rate of apoptosis
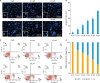 | Fig. 2PAB induces apoptosis in cervical cancer cells. HeLa cells were treated with different concentrations of PAB (0, 2.5, 5.0, 10, 20, 40 μM) for 24 hours. (A) Representative images of Hoechst 33258 staining of the nucleus (scale bar=200 μm; bottom left, scale bar=20 μm) (n=3). (B) The bar graphs represent the percentage of apoptotic cells in (A) (n=3, >30 fields per group were counted). (C, D) After AV/PI staining, percentage of cells in different apoptotic stages was analysed by flow cytometry (n=3). Data are expressed as mean values ± standard deviation for 3 independent experiments.AV, annexin V; FITC, fluorescein isothiocyanate; PAB, pseudolaric acid B; PI, propidium iodide.
*p<0.05 was considered significant.
|
3. PAB induces apoptosis through the mitochondrial pathway
 | Fig. 3PAB induces apoptosis in cervical cancer cell via the mitochondrial pathway. HeLa cells were treated with different concentrations of PAB (0, 2.5, 5.0, 10, 20, and 40 μM) for 24 hours to detect changes in mRNA expression levels and for 48 hours to detect changes in protein levels. (A) Relative expression levels of Fas, Caspase-8, and NIK mRNA in the Fas pathway (n=4). (B) Relative expression levels of caspase-3, CHOP, Caspase-12, and Calpain mRNA (n=4). (C) Relative expression levels of mitochondrial apoptosis-related gene mRNAs (n=4). (D-E) Relative expression levels of mitochondrial apoptosis-related proteins (n=4). (F-H) HeLa cells were subjected to mitochondrial and cytoplasmic separation, followed by measurement of CytC content in the mitochondria and cytosol (n=3). Values were normalised to β-actin in cytosol or COX IV in mitochondria. (I) Relative expression levels of Drp1, Fis1, Mfn1 and OPA1 mRNA (n=4). (J) Relative expression levels of BAX, Bcl-2 and Caspase-9 mRNA in cells treated with DMSO or Mdivi-1 (n=4). Data are expressed as mean values ± standard deviation.Bcl 2, B-cell lymphoma/leukemia-2; CHOP, C/EBP homologous protein; CytC, cytochrome; DMSO, dimethyl sulfoxide; Drp1, dynamin-related protein 1; Fis 1, fission protein 1; Mfn1, mitofusin 1; Mdivi-1, mitochondrial division inhibitor; NIK, NF-κB-inducing kinase; n.s., not significant; OPA 1, optic atrophy type 1; PAB, pseudolaric acid B.
*p<0.05 was considered significant.
|
4. PAB inhibits the expression of PAX2 and negatively regulates the transcription of BAX
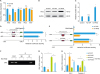 | Fig. 4PAX2 is inhibited by PAB and negatively regulates the expression of BAX. (A) Relative mRNA expression levels of the PAX family genes PAX2, PAX3, PAX5, PAX7, and PAX8 (n=4). (B, C) Efficiency of the adenoviral overexpression of PAX2 (n=4). (D) Dual luciferase reporter assay to investigate whether PAX2 binds to the BAX promoter: HEK293T were transfected with PGL3-basic (control), PGL3-BAX or PGL3-BAX-mutant plasmids (n=3). (E) Analysis of BAX promoter activity in HEK293T cells transfected with PGL3-basic (control), PGL3-BAX, or PGL3-BAX-mutant plasmids and infected with Ad-PAX2 (n=3). (F) Nuclear chromatin was collected from HeLa cells overexpressing PAX2, and subjected to ChIP assays using anti-HA or IgG antibodies. Another DNA input was collected as a positive control (n=3). (G, H) Relative mRNA expression levels of BAX, Bcl-2, and Caspase-3 with or without Ad-BAX (n=4). Data are expressed as mean values ± standard deviation.Bcl 2, B-cell lymphoma/leukemia-2; ChIP, chromatin immunoprecipitation; GFP, green fluorescent protein; IgG, immunoglobulin G; IP, immunoprecipitation; n.s., not significant; PAB, pseudolaric acid B; PAX2, paired box 2.
*p<0.05 was considered significant.
|
5. PAB-induced inhibition of apoptosis in HeLa cells is promoted by canonical Wnt signaling
 | Fig. 5PAB inhibits canonical Wnt signaling and promotes apoptosis in cervical cancer cells. (A) The protein interaction network of PAX2 was analysed online using STRING. The blue node represents PAX2, the green nodes represent proteins that interact with PAX2 in cancer pathways, and the red nodes represent the Wnt protein molecules in cancer signaling. (B, C) Effect of PAB on the protein levels of canonical and non-canonical Wnt signaling-related molecules (n=3). (D) TOPFlash-luc activity assays upon PAB treatment in HeLa cells (n=4). (E, F) Relative phosphorylation levels of GSK-3β and β-catenin and relative protein expression levels of cleaved Caspase-3 and PAX2 after DMSO, tideglusib or IWP-O1 treatment (n=3). Data are expressed as mean values ± standard deviation.DMSO, dimethyl sulfoxide; GFP, green fluorescent protein; IgG, immunoglobulin G; IP, immunoprecipitation; GSK-3β, glycogen synthase kinase-3β; n.s., not significant; PAB, pseudolaric acid B; PAX2, paired box 2.
*p<0.05 was considered significant.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download