This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
Highly effective chemotherapy for patients with low-risk gestational trophoblastic neoplasia (GTN) is associated with almost a 100% cure rate. However, 20%–30% of patients treated with chemotherapy need to change their regimens due to severe adverse events (SAEs) or drug resistance. We examined the treatment outcomes of second-line chemotherapy for patients with low-risk GTN.
Methods
Between 1980 and 2015, 281 patients with low-risk GTN were treated. Of these 281 patients, 178 patients were primarily treated with 5-day intramuscular methotrexate (MTX; n=114) or 5-day drip infusion etoposide (ETP; n=64). We examined the remission rates, the drug change rates, and the outcomes of second-line chemotherapy.
Results
The primary remission rates and drug resistant rates of 5-day ETP were significantly higher (p<0.001) and significantly lower (p=0.002) than those of 5-day MTX, respectively. Forty-seven patients (26.4%) required a change in their chemotherapy regimen due to the SAEs (n=16) and drug resistance (n=31), respectively. Of these 47 patients failed the first-line regimen, 39 patients (39/47, 82.9%) were re-treated with single-agent chemotherapy, and 35 patients (35/39, 89.7%) achieved remission. Four patients failed second-line, single-agent chemotherapy and eight patients (17.0%) who failed first-line regimens were treated with combined or multi-agent chemotherapy and achieved remission.
Conclusions
Patients with low-risk GTN were usually treated with single-agent chemotherapy, while 20%–30% patients had to change their chemotherapy regimen due to SAEs or drug resistance. The second-line regimens of single-agent chemotherapy were effective; however, there were several patients who needed multiple agents and combined chemotherapy to achieve remission.
Keywords: Low-risk Gestational Trophoblastic Neoplasm, Drug Resistance, Chemotherapy
INTRODUCTION
Gestational trophoblastic neoplasia (GTN) is extremely responsive to chemotherapy, and cure rates of 100% have been achieved with chemotherapy alone for patients with low-risk GTN. The most common and effective chemotherapy regimen for patients with low-risk GTN is single-agent chemotherapy using methotrexate (MTX), actinomycin-D (Act-D), or etoposide (ETP) [
12345].
Although the MTX regimen has several dosing, cycling, and route options, with or without folinic acid rescue, it is effective as a first-line drug for low-risk GTN due to its favorable safety and toxicity profile. However, approximately 30% of patients with low-risk GTN who were treated with MTX (with or without folinic acid rescue) require a change in their chemotherapy regimen due to the development of drug resistance and/or intolerable toxicity, which includes severe stomatitis, an increase in aspartate aminotransferase levels, and severe drug eruption [
1234].
The Act-D regimen has both a pulsed or 5-day intravenous injection (5-day Act-D) options. Adverse events due to Act-D regimen include alopecia and moderate to severe nausea and vomiting. Moreover, skin necrosis due to an extravasation of Act-D is an irreversible and terrible adverse event. Osborne et al. [
5] reported that 27% (29/109) of patients with low-risk GTN treated with pulsed Act-D developed drug resistance and required changes in their regimens.
In 1979, Newlands and Bagshawe [
6] first reported that ETP was highly effective in the treatment of GTN. Adverse events of ETP were alopecia, moderate to severe nausea and vomiting, ovarian dysfunction, and the possibility of secondary malignancy after combination chemotherapy containing ETP [
78910].
In this study, we retrospectively analyzed the primary remission rates, relapse rates, drug resistance, and drug toxicity rates among patients with low-risk GTN who were treated with first-line, single-agent chemotherapy with a 5-day low-dose intramuscular MTX (5-day MTX) or a 5-day intravenous drip ETP infusion (5-day ETP). Furthermore, we also examined the choices and outcomes of second-line chemotherapy regimen in patients with low-risk GTN who developed drug resistance and drug toxicity.
MATERIALS AND METHODS
Between 1980 and 2015, 281 patients with low-risk GTN (International Federation of Gynecology and Obstetrics [FIGO] score <7) were treated at Chiba University (1980–2009) and Tokyo Women's University (2010–2015). The design for this retrospective study was reviewed and approved by our institutional ethics review board. The pre-treatment evaluation of each patient included a complete history and physical examination, complete blood count, renal and liver function tests, serum human chorionic gonadotropin (hCG) levels, pelvic ultrasonography, and chest radiography and/or lung computed tomography. Serum hCG values were evaluated before each chemotherapy cycle and twice per week during the chemotherapy cycle.
Of these 281 patients with low-risk GTN, 103 patients (36.7%) were excluded in this analysis due to the combined planned hysterectomy and chemotherapy (n=72); initial treatments with several combinations of chemotherapy (n=13); and the administration of other single-agent regimen (5-day Act-D; 6 patients, 8-day MTX-CF; 12 patients). The remaining 114 patients (40.6%) and 64 patients (22.4%) were primarily treated with 5-day MTX (0.4 mg/kg intramuscularly) or 5-day ETP (2.0 mg/kg divided), respectively (
Fig. 1). These regimens were repeated every 10–14 days until the serum hCG levels decreased to the normal range [
11]. Forty-seven patients (26.4%) had to change their chemotherapy regimens due to severe adverse events (SAEs; n=16) and the development of drug resistance (n=31), respectively. Furthermore, we examined the outcomes of second-line chemotherapy.
Fig. 1
Flow diagram and the patients' characteristics of all 281 patients.
Act-D, actinomycin-D; CF, citrovorum factor; ETP, etoposide; FIGO, International Federation of Gynecology and Obstetrics; GTN, gestational trophoblastic neoplasia; MTx, methotrexate.

There is no internationally-accepted consensus definition for drug resistance due to chemotherapy; therefore, patients were defined as developing drug resistance if they reached a plateau (<50% decrease in their hCG titers) for ≥2 consecutive cycles of chemotherapy, or if they showed an increase in hCG levels during chemotherapy cycles. Drug toxicity profiles were classified according to the Common Terminology Criteria for Adverse Events (CTCAE version 4.0; National Cancer Institute, Rockville, MD, USA) [
12]. Cases with at least grade 3 stomatitis; at least a grade 2 increase in aspartate aminotransferase levels; or at least grade 1 skin rash (macules, papules) were diagnosed as having SAEs and their chemotherapy regimen was changed. Primary remission was defined as three consecutive weekly hCG levels that were within the normal range using the same chemotherapy regimen.
All data were reported as mean±standard deviation, median (range), or number (%). Continuous data were examined for skewness and kurtosis using JMP Pro software (version 11.2.0; SAS, Inc., Japan, Tokyo). Proportional data were analyzed using Fisher's exact test. Differences with a p-value of <0.05 were considered statistically significant.
RESULTS
The primary remission rates, drug changing rates, and relapse rates of 178 patients with low-risk GTN primarily treated with 5-day MTX or 5-day ETP are shown in
Table 1. Primary remission rates of patients treated with 5-day MTX or 5-day ETP were 64.0% (73/114) and 90.6% (58/64), respectively. The primary remission rates of patients treated with ETP were significantly higher than in patients treated with 5-day MTX (p<0.001). The need for drug changes of patients treated with 5-day MTX was significantly higher than those in patients treated with 5-day ETP, with respect to toxicities (p=0.032) and drug resistance (p=0.002), as previously reported [
311]. Relapse rates with 5-day MTX and 5-day ETP were 1.8% (2/114) and 1.6% (1/64), respectively.
Table 1
Primary remission rates, drug resistance rates, drug toxicity, and relapse rates

|
Regimen |
No. of patient |
Primary remission rate |
p |
Regimen change |
Relapse rate |
p |
|
Toxicity |
p |
Resistance |
p |
|
5-day MTX |
114 |
73 (64.0) |
<0.0001 |
14 (12.3) |
0.032 |
27 (23.7) |
0.002 |
2 (1.8) |
0.775 |
|
5-day ETP |
64 |
58 (90.6) |
<0.0001 |
2 (3.1) |
0.032 |
4 (6.3) |
0.002 |
1 (1.6) |
0.775 |
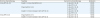
Fourteen patients treated with 5-day MTX required a change from the MTX regimen due to grade 3 or more stomatitis (n=5), drug eruption (n=5), and a grade 2 increase in aspartate aminotransferase levels (n=4) after a median of 1 cycle of MTX (range, 1 to 5 cycles). The regimen was changed to 5-day Act-D (n=7) and 5-day ETP (n=7), respectively. Two patients treated with 5-day ETP also required a change in ETP regimen due to drug eruption and a grade 2 increase in aspartate aminotransferase levels after 1 cycle of ETP, respectively. The chemotherapy regimen was changed to 5-day Act-D. All 16 patients who experienced adverse events from MTX or ETP achieved remission with second-line, single-agent chemotherapy (
Table 2).
Table 2
Drug toxicities of first-line regimens and outcomes of second-line regimens
|
First-line chemotherapy |
Toxicities |
Second-line chemotherapy |
Remission |
|
5-day MTX (n=14) |
Grade 3 or more stomatitis (n=5) |
5-day Act-D (n=2) |
2 (100) |
|
5-day ETP (n=3) |
3 (100) |
|
Drug eruption (n=5) |
5-day Act-D (n=3) |
3 (100) |
|
5-day ETP (n=2) |
2 (100) |
|
Grade 2 or more increase in GOT, GPT (n=4) |
5-day Act-D (n=2) |
2 (100) |
|
5-day ETP (n=2) |
2 (100) |
|
5-day ETP (n=2) |
Drug eruption (n=1) |
5-day Act-D (n=1) |
1 (100) |
|
Grade 2 or more increase in GOT, GPT (n=1) |
5-day Act-D (n=1) |
1 (100) |
Twenty-seven patients (23.7%) treated with 5-day MTX also required a change in regimen due to the development of drug resistance after a median of 4 cycles of MTX (range, 1 to 9 cycles). The second regimens after the failure of 5-day MTX were 5-day Act-D (n=10), 5-day ETP (n=12), classical combined regimen of MTX and Act-D (n=3), dose escalation MTX (n=1), or a multiple-agent regimen composed of MTX, ETP, and Act-D (MEA; n=1) [
13], respectively (
Table 3).
Table 3
Outcomes of second-line regimens in patients with drug resistance

|
First-line regimen |
Second-line regimen |
No. of cases |
Remission |
Drug resistance |
|
5-day MTX (n = 27) |
5-day Act-D |
10 |
8 (80.0) |
2 (20.0) |
|
5-day ETP |
12 |
10 (83.3) |
2 (17.7) |
|
MTX dose escalation |
1 |
1 |
0 |
|
Combined MTX and Act-D |
3 |
1 (33.3) |
2 (66.7) |
|
MEA |
1 |
1 |
0 |
|
5-day ETP (n = 4) |
Combined ETP and Act-D |
3 |
3 |
0 |
|
MEA |
1 |
1 |
0 |
|
Total |
|
31 |
25 (80.6) |
6 (19.4) |
Eight patients (80%) who were treated with second-line chemotherapy of 5-day Act-D regimen achieved remission with a mean of 3.25 cycles of Act-D (range, 2 to 5 cycles), while two patients showed a failure to respond to the second-line regimen of 5-day Act-D. These 2 patients were salvaged with the combined regimen and MEA, respectively. Ten patients (83.3%) treated with the second line regimen of 5-day ETP achieved remission with a mean of 4.2 cycles of 5-day ETP (range, 2 to 8 cycles). Two patients showed a failure to respond to the second-line 5-day ETP regimen, and they achieved remission with the combined chemotherapy regimen.
One patient treated with combined MTX and Act-D achieved remission, while 2 patients showed drug resistance to the second-line regimen of combined MTX and Act-D. These 2 patients achieved remission with multiple-agent combination chemotherapy.
The other 2 patients with drug resistance to the first-line regimen of 5-day MTX were treated with MEA and an increased dose of MTX. The clinical course of a patient treated with MEA regimen was as follows. The patient was referred to our clinic 19 weeks after mole evacuation. She showed left lung metastasis (14×10 mm). Her pre-treatment hCG titers were 2,860 mIU/mL, and FIGO score was 5. She was initially treated with 5-day MTX and her hCG titers constantly declined to 3.4 mIU/mL after 3 cycles of MTX. However, her hCG titers showed a plateau during the next 3 cycles of MTX. Moreover, her hCG titers increased to 16.8 mIU/mL at the start of the seventh cycle of MTX. After metastatic analyses, she was treated with MEA regimen and attained hCG titers below cut-off levels after 5 cycles of MEA.
Four patients primary treated with 5-day ETP developed drug resistance. Three of them were treated with combined ETP and Act-D regimen and the remaining patient was treated with MEA regimen. The clinical course of a patient treated with a second-line regimen of MEA was followed. She was referred to our clinic at 6 weeks after mole evacuation. Her pre-treatment hCG titers were 39,600 mIU/mL, and right lung metastasis (5×3 mm) and uterine lesions (12×10 mm) were found. Her FIGO score was 3. She was treated with 5-day ETP and her hCG titers did not decline (14,700 mIU/mL) after 4 cycles of 5-day ETP. A second-line regimen of MEA was started and hCG levels were attained under cut–off levels after 8 cycles of MEA. All 4 patients with a failure of 5-day ETP can be salvaged with these second-line regimens.
Three relapsed cases were observed in this analysis. Two relapsed patients were primarily treated with 5-day MTX. They had no lung metastases as revealed by chest computed tomography. The pre-treatment hCG titers were 64,000 and 490 mIU/mL, and FIGO scores were 2 and 5, respectively. They achieved remission with 6 and 8 cycles of 5-day MTX; however, they relapsed 3 and 3.5 months after the last 5-day MTX chemotherapy, respectively. Another relapse case was primarily treated with 5-day ETP. She had lung metastases as shown through chest computed tomography, the pre-treatment hCG was 36,952 mIU/mL, with a FIGO score of 4. She was treated with 9 cycles of 5-day ETP. After this, she exhibited drug resistance to 5-day ETP and was switched to combined ETP and Act-D regimen. She achieved remission with 4 cycles of the second regimen, but she relapsed 5 months after the last chemotherapy.
All 3 relapsed patients achieved remission with the MEA regimen.
DISCUSSION
The curative rates of low-risk GTN have approached 100% with the introduction of effective chemotherapy regimens, even in the presence of metastasis, and a failure to respond to first-line, single-agent chemotherapy regimens [
14]. MTX, Act-D, and ETP are the most common and effective drugs for patients with low-risk GTN [
12345], however, there is no consensus concerning which drugs and regimens are optimal for patients with low-risk GTN [
1516]. In our hospitals, 5-day ETP was preferred as the first-line chemotherapy regimen for patients with low-risk GTN between 1986 and 2003, while 5-day MTX tends to be preferred at present due to several unfavorable adverse events in young women of reproductive age, including alopecia and ovarian dysfunction associated with ETP [
10].
Approximately 20%–30% of patients treated with first-line, single-agent chemotherapy required a change in their regimen due to SAEs or drug resistance [
51415]. Soper et al. [
17] reported that primary remission was achieved in 60% (31/52) of patients treated with 5-day MTX, while 21 patients required second-line chemotherapy due to drug resistance (10 patients) and SAEs (11 patients). They also found that high pre-treatment hCG levels were associated with drug resistance. In our previous analysis [
11], similar remission rates (66/102, 64.7%) and drug resistance rates (23/102, 22.6%) from 5-day MTX were reported; however, overall survival was 100%.
McNeish et al. [
1] reported a similar remission rate (324/485, 66.8%) and drug resistance rate (150/485, 30.9%) of low-risk GTN treated with 8-day MTX-FA regimen from the data of Charing Cross Hospital. Moreover, authors stated that second-line chemotherapy regimen was altered according to the hCG levels at the time of developing drug resistance and toxicity. If serum hCG levels were approximately ≤100 IU/L, second-line chemotherapy was changed to a single-agent Act-D regimen and 86.6% (58/67) of patients achieved remission. If hCG was greater than 100 IU/L, second-line chemotherapy was changed to multi-agent chemotherapy of ETP, methotrexate and actinomycin D alternating weekly with cyclophosphamide and vincristine (EMA/CO) and 98.9% (93/94) of patients achieved remission. In this report, 21.2% (103/485) of patients with low-risk GTN were treated with multi-agent combination chemotherapy containing ETP and cyclophosphamide for second-line or third-line regimen. ETP was reportedly associated with an increased risk of secondary tumors including leukemia, breast cancer, colon cancer, and melanoma. The risk of second malignancies might be high in these low-risk GTN patients treated with multi-agent chemotherapy.
Recently, McGrath et al. [
18] reported that low-risk GTN patients whose pre-treatment hCG >400,000 IU/L should receive multi-agent chemotherapy (EMA/CO) from the onset of treatment due to the poor outcomes of the MTX-FA regimen.
In this analysis, 14 patients (12.3%) treated with 5-day MTX and 2 patients (3.1%) treated with 5-day ETP required a change from the first-line regimen due to SAEs. All these 16 patients achieved remission with second-line, single-agent chemotherapy. Of 31 patients with drug resistance to 5-day MTX or 5-day ETP, 74.2% (23/31) were treated with second-line, single-agent chemotherapy and 82.6% (19/23) achieved remission. Twelve patients (12/180, 6.7%) were treated with second-line or third-line combination regimens (MTX and Act-D, or ETP and Act-D) or a multi-agent combination chemotherapy containing ETP or cyclophosphamide. In our treatment, several ETP-containing regimens have been used, including the first-line 5-day ETP (n=64), the second-line 5-day ETP (n=19), second-line and third-line combined 5-day ETP and Act-D (n=4), and MEA (n=5). Fortunately, no leukemia was observed.
In conclusion, single-agent chemotherapy using 5-day MTX or 5-day ETP was effective for patients with low-risk GTN, although approximately 35% of patients treated with 5-day MTX and approximately 10% of patients treated with 5-day ETP require a change in their regimen due to SAEs or drug resistance. Patients with SAEs were treated with second-line single-agent regimens and all patients were salvaged. Patients with drug resistance were treated with several kinds of chemotherapy regimens, while 74.2% of patients (23/31) were again treated with a single-agent chemotherapy regimen and 83% of patients were salvaged. Second-line multi-agent chemotherapy is needed when hCG titers during chemotherapy show a plateau or increase during the first-line regimen, or the patient exhibits a failure to respond to second-line regimen for any reason.








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download