Abstract
The phrase "locally advanced carcinoma of the vulva" has often been mentioned in the literature, though not accurately defined, or even leading to the interpretation overlapping. Grounded on cervical cancer experience, we are able to state that designing a tailored primary strategy based on clinically measurable adverse prognostic factors represents the cornerstone of therapy. This fact urged us to rethink about the real usefulness of the concept of locally advanced squamous cell carcinoma of the vulva. We will refer to this concept as a clinical entity emerging from a rigorous workup which is a valuable guiding tool in the context of a thorough debate about the best primary treatment approach to be used. Furthermore, bulky tumors of the vulva have been associated with a worse prognosis on several occasions. Some authors have questioned the fact that tumor size has not been considered in the staging system. Finally, a standardized definition will help us compare the results obtained, which is extremely necessary given the worldwide low prevalence of this disease.
It is estimated that one third of the patients with squamous cell carcinoma of the vulva (SCCV) will be diagnosed at the time of surgical unresectability, a setting which has historically been defined as "locally advanced disease" [1]. This entity features either close or overt involvement of neighboring organs such as the vagina, urethra, vesical mucosa, anus and/or rectum and eventually the tumor gets fixed to the bone. Besides, the association with widespread inguinofemoral metastasis is not uncommon. Therefore, it is clear to see that the surgical approach including standard radical vulvectomy and bilateral groin dissection is not feasible, at least initially. Candidate selection for a particular type of treatment strategy will depend not only on the location and size of the tumor but also on performance status, and the general characteristics of the patient. The critical treatment aims at present are to maximize tumor control, and minimize both functional and cosmetic damage which may occur after treatment. SCCV is not related to other histological variations, and therefore it may be described as a separate nosological entity. Nonsquamous cell carcinomas of the vulva such as melanomas, basal cell carcinomas, and adenocarcinomas represent a heterogeneous minority of histological types to be assessed separately since its biology, evolution, and prognosis are different.
However, the concept of a locally advanced squamous cell carcinoma of the vulva (LASCCV) has not been accurately described in the world medical literature and even sometimes in a contradictory fashion or leading to the overlapping of interpretations. Different factors may account for this fact:
In the first place, unless clearly detailed, the concept of surgical unresectability is subjective in nature, and largely depends on the surgical ideology of the surgeon, and the cost they will bear in terms of postoperative morbidity and mortality. In many papers published on this topic no clear reference has been reported on either what the authors mean by "unresectability" or the failure to achieve a given surgical margin or what such margin should be.
In the second place, a strategy with a curative intention to "convert the unresectable" into "potentially resectable" conceptually differs from "an intent to improve the locoregional conditions of surgical resectability" with the only aim to perform smaller resections and/or avoiding partial resection of neighboring organs, when this group of patients may be treated with primary radical surgery and achieve appropriate surgical margins.
Finally, even when the percentage of patients diagnosed in "advanced stages" (III to IV) as defined by the staging system of the International Federation of Gynecology and Obstetrics (FIGO) established in 1988 has been estimated in 30% to 40% [2], a figure which matches the percentage of patients diagnosed with "locally advanced disease," it is not always the case for patients who meet both definition criteria. In fact, the FIGO system has never made any reference to whether involved neighboring structures may be resectable or not or to which is the best strategy to achieve resectability, or else to the sparing of physiological function of the organs involved. Moreover, the 1988 system did not take into account whether the nodes were fixed and/or ulcerated, that is, potentially unresectable. These two subgroups of patients become even more separated after the latest modification in 2009 where stage III is used only to describe the histological status of the nodes [3]. The only parallelism that remains with LASCCV is IVA. If we consider that IVA stage now represents 3.2% of the total, due to the downstaging resulting from the switching of patients to a new stage III [4], we are now far from the initial 30% to 40%. Consequently, these concepts should not be confused: the FIGO staging system includes a histological analysis of the surgical specimen of patients who may have an indication for adjuvant radiotherapy, and then the prognosis is based on survival chances, whereas the concept of LASCCV refers to a clearly clinical entity, when the patient has not even been treated, and is a guiding tool in a thorough debate about the therapy approach to be used, which in most cases does not include primary radical surgery, as mentioned above.
The interesting debate is whether those with stage II must be considered locally advanced. We do not think they should. The fact that these patients are candidates for primary resection achieving adequate surgical radicality brings about a conflict with the concept of LASCCV. Scientific evidence supports the above. Resections of as much as 1.5 cm of the distal urethra to obtain negative surgical margins do not seem to involve vesical continence [5], tumors involving the anus or the anal sphincter may be managed with wide local resection and sphincter repair or by means of flaps as a valid option to chemoradiation or ultraradical therapy; in these cases reconstruction may lead to complete continence and proper surgical radicality [6]. Oncoplasty should be considered as an important resource in gynecological radical surgery. Finally, these patients may also be successfully managed with neoadjuvant chemotherapy (NCH), achieving high response rates and improved surgical feasibility-which allows neighboring structure sparing [7]-and avoiding the untoward toxic effects of radiotherapy. Based on the good prognosis of patients with node-negative SCCV involving the vagina and/or urethra and following the suggestions of several authors, the old stage III has now become stage II. The present stage III now is not related to chances of surgical resection of the disease. Finally, this might lead to a reassessment of the truly "advanced stages" in SCCV.
Attempts have been made at new definitions in the last 60 years, ranging from metastasic disease involving bones or the lungs as the only criterion of unresectability, to anus, anal sphincter, rectum, rectovaginal septum, or proximal urethra involvement, being pelvic exenteration combined with radical vulvectomy and bilateral inguinofemoral lymphadenectomy the only chance for a cure, and most recently, a widespread but tailored radical vulvectomy which consists of partial resection of neighboring organs strictly depending on cancer spread. The development of neoadjuvant radiotherapy and then neoadjuvant concurrent chemoradiotherapy (CCHR) followed by radical surgery in the management of advanced disease meant significant advances. The Gynecologic Oncology Group (GOG) trial #101 (2000), introduced the interesting concept of "advanced nodal disease," defined as unresectable regional nodes [8]. Some literature reviews have been conducted based on comparisons between neoadjuvant CCHR followed by radical surgery and radical surgery alone in order to come to conclusions validating different approaches. However, questionable methodologies, unclear selection criteria and the dichotomy between patients with "operable advanced disease" and "inoperable advanced disease" make the interpretation of results difficult. In this respect, a Cochrane review (2011) on this subject concluded that no standard terminology is available for either "operable and inoperable vulvar cancer" or "primary and neoadjuvant CCHR" after reviewing most important papers in this field, and for a good quality comparison staging based on unresectability of the primary tumor and/or primary lymph nodes is necessary [9]. The debate about the best therapeutic option is still ongoing.
It has been known for more than seventy years that if node resection is not possible, the disease will inevitably progress. So, it is highly encouraging to think that if necessary conditions to achieve resectability may somehow be achieved, the patient would then undergo a potentially curative surgical procedure. This was first described by the GOG [8]. For this reason, we consider that the clinical conditions of inguinal nodes must be taken into account and included in the LASCCV concept. The presence of unresectable nodes leads to a reassessment of the initial treatment strategy, even when the primary tumor may be clearly resectable. These options as such are suggestive of the need for an integrating concept.
We define LASCCV as a clinical presentation of the disease, without distant metastasis, when primary treatment with radical surgery is not feasible due to the presence of unresectable disease; that is, the impossibility to remove the tumor with adequate surgical margins, and consequently the need to use neoadjuvant therapy, primary CCHR, or else, ultraradical surgery. Inguinofemoral nodes are included in this definition when they are fixed to fascia, muscle, or vascular structures.
The clinical staging system for vulvar cancer designed in the 70s by FIGO adopted 2 cm of tumor diameter as the cutoff point [10] based on the fact that over that value, tumors are highly likely to present positive nodes, a concept that was later studied and confirmed by several authors [11,12]. However, this system was criticized since it provided reliable information about the primary tumor but a 20% to 30% error margin in terms of node assessment, with a poor correlation between the clinical node status and the histological findings [13,14]. Back in 1977, it was suggested that the system should be replaced by another one to make up for deficiencies [15]. Since both local and regional surgery represent the cornerstone in the management of SCCV, the histological node status was included in the staging system and so in 1988 it became surgical [16]. Consequently, the presence of positive nodes, placed these patients in a more advanced stage, and the prognosis was then defined by this condition. However, not all the tumors >2 cm are necessarily associated to a poor prognosis. Several authors have studied other cutoff points, which seem to adequately correlate with the prognosis, even in patients with negative nodes (Table 1) [4,17,18,19,20,21,22,23,24,25]. In fact, after the changes made in 1988, no significant differences were seen in terms of survival either between stages I and II: 98% vs. 85%, or between stages II and III: 85% vs. 74%; in the latter case survival rates have been surprisingly high [26]. Patients with stage III disease had a wide spread of survival rates (34% to 100%), including a really heterogeneous group of patients [27] when ideally, the survival for the four major FIGO stages should be reasonably evenly spread between 0% and 100%. New FIGO staging does not solve this problem as it has grouped prior 1988 stages I and II into a single stage I, minimizing even more the effect of size on prognosis when the lesion is confined to the vulva or perineum [3]. Considering that the prognosis of the current stage IB is determined by tumor size (>2 cm), an overlapping of prognostic factors may be expected if we think that the prognosis of a patient with a primary tumor of 3 cm is identical to that of a patient with a primary tumor >8 cm when the disease does not involve neighboring organs and has negative nodes. The same reasoning may be applied to those with stage II which is mainly true if we consider that the involvement of neighboring structures, such as the vagina, lower urethra, and anus (formerly stage II) has not proved to be a relevant independent prognostic factor when adequate surgical margins are obtained [21,28]. Finally, some authors have questioned the similarity related to survival rates in stages I and IIIA [29].
As a first conclusion, the FIGO staging system does not stage survival well between stages I and II, reducing survival in stage I cases by including larger lesions. We agree with Tabbaa et al. [4] that patients in stages II might be subdivided considering tumor size, which may be even more important than neighboring structure involvement. Regarding the latter, we would like to express our concern based on the relationship between patients with large tumors of the vulva and a really more torpid outcome. This is so even when the presentation of the disease includes favorable conditions for resectability at the vulvoperineal level, and histologically negative nodes. In our working environment, due to the social and cultural situation, about 70% of the patients present for the first time already with tumors >6 cm. We have recently published the results of a study with the aim to assess those known prognostic factors in primary SCCV, including 194 patients undergoing radical primary surgery with adequate margins in all the cases with a median follow-up of 68 months [25]. This trial clearly identified a high risk group for failure to survive including patients with a tumor size between ≥6 to 7.9 cm and a depth of stromal invasion (DSI) >4 mm or with a tumor size ≥8 cm irrespective of DSI. This new bulky tumor high-risk group presented a 5-year overall survival rate of 24%. Patients with extra-nodal growth or ≥2 positive lymph nodes, in both cases irrespective of tumor size and DSI, also belonged to this group. On analyzing separately the stage II, survival declined from 87% for tumors between 2.1 and 3.99 cm to around 50% for tumors between 4 and 7.99 cm. Furthermore, our trial reported an interesting subgroup of patients in stages IB and II included in our high risk group (representing 18% of the group), and presented adverse prognosis in spite of belonging to "early FIGO stages." Since these surgically managed patients have even poorer survival rates than patients with surgically unresectable disease treated with chemoradiation [30], we are forced to rethink the LASCCV definition, but now including primary tumor size, as well as to reconsider all the therapeutic strategies for bulky vulvar cancer management available to date. Our trial is not the first to describe a high risk group in SCCV (Table 1) [19,21]. So, the question is the following: are we recruiting our patients adequately according to their chances of survival? Furthermore, are we offering tailored treatment accordingly? Once again, the concept of LASCCV should be reviewed.
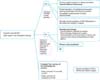
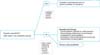
An ongoing prospective randomized phase III trial is being carried out at the Gynecologic Oncology Department of the Oncology Hospital of Buenos Aires Marie Curie comparing NCH followed by radical surgery vs. primary radical surgery in this high risk group of patients. Our current recommendations for the management of LASCCV appear in Figs. 1, 2.
Tumor size in SCCV may be effectively predicted with clinical assessment and a high clinical-pathological correlation may be obtained, leading then to a tailored primary strategy. The same situation applies to cervical cancer. As we stated above, it has been long known that together with the node status, tumor size represents an essential prognostic factor in cervical cancer and although not all authors have agreed on the value of the cutoff point to be adopted, strong evidence supports the fact that over a "certain critical tumor mass" survival rates decrease remarkably [31,32,33,34,35]. A bulky tumor in cervical cancer was addressed for the first time by the FIGO Committee on Gynecologic Oncology in Montreal (1994) as a lesion with a maximum diameter >4 cm [30]. Thus, the entity of 'bulky' stage IB (IB2) was first added in 1994 [36] and lately the entity of "bulky" stage IIA (IIA2) in the FIGO last modification carried out in 2009 [3]. Therefore, as opposed to SCCV, tumor size is included in the stage subdivision. The FIGO committee felt this division of stage IB into IB1 and IB2 was a step to try and further delineate a wide spectrum of disease to then determine the best treatment modality. Besides, vulvar stages IA and IB were also modified, but in this case, the effect of such modification had very little impact on survival. This is easy to understand, for although depth of invasion predicts lymph node metastasis, if lymph nodes are positive the patient no longer has stage I cancer [36].
The optimal treatment of bulky stages-that is IB2 and IIA >4 cm (currently IIA2), and locally-advanced stages, defined as stage IIB, III, and IVA in both cases accordingly to FIGO definitions [37]-has remained a controversial issue. Despite improvements in treatments through the years, a main point is that 5-year overall survival rates continue to be low for patients with locally advanced squamous cell carcinoma of the cervix (LASCCC) treated with primary CCHR [38,39] and for patients with LASCCV treated with neoadjuvant CCHR [9,40], both considered standard treatments today. A possible explanation may be that voluminous lesions present a large hypoxic tumor cell population that reduces radiosensitivity. The latter seems to be applicable to squamous gynecological solid tumors in general.
One could argue that making a comparison between SCCV and cervical cancer is unnecessary or irrelevant because of the differences related to biological and demographical characteristics. However, from a prognostic standpoint, when squamous cell carcinomas of the female lower genital tract have a tendency to exophytic or expansive growth, these differences begin to clear away as survival rates worsen, even with treatments considered standard. Therefore, the bulky tumor concept from the point of view of a "critical tumor mass volume" encourages us to perform a thorough analysis in terms of which treatment modality to adopt. In the case of cervical cancer, although the debate is still going on as to whether the cutoff point for a tumor to be considered bulky should be 3, 4, 5, or 6 cm, and which primary treatment is the best option; the concept as such is here to stay. This is not so in the case of SCCV, where a heated debate is still to be conducted. The importance of this debate is such that we might design even better and more customized primary treatments.
We sustain a definition of radical vulvar surgery including resection of neighboring organs such as the vagina, anus, and/or proximal urethra if necessary to achieve adequate surgical radicality ("tailored radical vulvectomy"). As explained above, patients with stage II have a good prognosis and several options to decrease the comorbidity rate associated with treatment.
Ultraradical vulvar surgery refers to pelvic exenteration type III according to the classification by Magrina et al. [41]. There is ample recent evidence that pelvic exenteration still today plays a role in terms of cure in these patients and that the chances of being successful are much greater if correct selection criteria are applied [42].
Based on available scientific evidence, we assume a pathological tumor-free margin of at least 8 mm after formalin fixation as a standard recommendation [43]. We have read with great interest the publications by Chan et al. [43,44] on resection of primary tumors with macroscopic margins smaller than those historically recommended and encouraging results. We are following the impact that this concept may have, and therefore, about increasingly conservative surgeries.
Our belief is that tumor size must be taken into account when deciding on SCCV treatment approach. Extrapolation of study results from LASCCC could be seen as a contribution to improve our knowledge of LASCCV.
We believe the definition of LASCCV should be used only in treatment naive patients. In the case of previous treatment with oncological radical criteria the terms local relapse, locoregional relapse, skin metastasis, etc., should be used.
A standardized definition will help us compare the results obtained, which is extremely necessary given the worldwide low prevalence of this disease.
The individualization of cancer therapy can only be achieved by an exhaustive knowledge of biological and presurgical conditions that could perfect primary strategies and therefore have a direct impact on patients' chances of survival.
Figures and Tables
Fig. 1
Authors recommendations for the management of probably locally advanced squamous cell carcinoma of the vulva (LASCCV): primary treatment of local disease. DSI, depth of stromal invasion; NCH, neoadjuvant chemotherapy; PE, pelvic exenteration.
References
1. Daily LJ, Kaplan AL, Kaufman RH. Exenteration for advanced carcinoma of the vulva. Obstet Gynecol. 1970; 36:845–849.
2. Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER cancer statistics review, 1975-2008. Bethesda, MD: National Cancer Institute;2012.
3. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009; 105:103–104.
4. Tabbaa ZM, Gonzalez J, Sznurkowski JJ, Weaver AL, Mariani A, Cliby WA. Impact of the new FIGO 2009 staging classification for vulvar cancer on prognosis and stage distribution. Gynecol Oncol. 2012; 127:147–152.
5. de Mooij Y, Burger MP, Schilthuis MS, Buist M, van der Velden J. Partial urethral resection in the surgical treatment of vulvar cancer does not have a significant impact on urinary continence: a confirmation of an authority-based opinion. Int J Gynecol Cancer. 2007; 17:294–297.
6. Barton DP, Hoffman MS, Roberts WS, Fiorica JV, Finan MA, Gleeson N, et al. Use of local flaps in the preservation of fecal continence following resection of perianal neoplasias. Int J Gynecol Cancer. 1993; 3:318–323.
7. Aragona AM, Cuneo N, Soderini AH, Alcoba E, Greco A, Reyes C, et al. Tailoring the treatment of locally advanced squamous cell carcinoma of the vulva: neoadjuvant chemotherapy followed by radical surgery: results from a multicenter study. Int J Gynecol Cancer. 2012; 22:1258–1263.
8. Montana GS, Thomas GM, Moore DH, Saxer A, Mangan CE, Lentz SS, et al. Preoperative chemo-radiation for carcinoma of the vulva with N2/N3 nodes: a gynecologic oncology group study. Int J Radiat Oncol Biol Phys. 2000; 48:1007–1013.
9. Shylasree TS, Bryant A, Howells RE. Chemoradiation for advanced primary vulval cancer. Cochrane Database Syst Rev. 2011; (4):CD003752.
10. Classification and staging of malignant tumours in the female pelvis. Acta Obstet Gynecol Scand. 1971; 50:1–7.
11. Franklin EW 3rd. Clinical staging of carcinoma of the vulva. Obstet Gynecol. 1972; 40:277–286.
12. Morley GW. Cancer of the vulva: a review. Cancer. 1981; 48:2 Suppl. 597–601.
13. Homesley HD, Bundy BN, Sedlis A, Yordan E, Berek JS, Jahshan A, et al. Prognostic factors for groin node metastasis in squamous cell carcinoma of the vulva (a Gynecologic Oncology Group study). Gynecol Oncol. 1993; 49:279–283.
14. Podratz KC, Symmonds RE, Taylor WF, Williams TJ. Carcinoma of the vulva: analysis of treatment and survival. Obstet Gynecol. 1983; 61:63–74.
15. Friedrich EG Jr, diPaola GR. Postoperative staging of vulvar carcinoma: a retrospective study. Int J Gynaecol Obstet. 1977; 15:270–274.
16. Announcements: FIGO stages 1998 revision. Gynecol Oncol. 1989; 35:125–127.
17. Krupp PJ, Lee FY, Bohm JW, Batson HW, Diem JE, Lemire JE. Prognostic parameters and clinical staging criteria in the epidermoid carcinoma of the vulva. Obstet Gynecol. 1975; 46:84–88.
18. Podratz KC, Symmonds RE, Taylor WF. Carcinoma of the vulva: analysis of treatment failures. Am J Obstet Gynecol. 1982; 143:340–351.
19. Andreasson B, Nyboe J. Value of prognostic parameters in squamous cell carcinoma of the vulva. Gynecol Oncol. 1985; 22:341–351.
20. Rutledge FN, Mitchell MF, Munsell MF, Atkinson EN, Bass S, McGuffee V, et al. Prognostic indicators for invasive carcinoma of the vulva. Gynecol Oncol. 1991; 42:239–244.
21. Homesley HD, Bundy BN, Sedlis A, Yordan E, Berek JS, Jahshan A, et al. Assessment of current International Federation of Gynecology and Obstetrics staging of vulvar carcinoma relative to prognostic factors for survival (a Gynecologic Oncology Group study). Am J Obstet Gynecol. 1991; 164:997–1003.
22. Paladini D, Cross P, Lopes A, Monaghan JM. Prognostic significance of lymph node variables in squamous cell carcinoma of the vulva. Cancer. 1994; 74:2491–2496.
23. Kirschner CV, Yordan EL, De Geest K, Wilbanks GD. Smoking, obesity, and survival in squamous cell carcinoma of the vulva. Gynecol Oncol. 1995; 56:79–84.
24. Smyczek-Gargya B, Volz B, Geppert M, Dietl J. A multivariate analysis of clinical and morphological prognostic factors in squamous cell carcinoma of the vulva. Gynecol Obstet Invest. 1997; 43:261–267.
25. Aragona AM, Cuneo NA, Soderini AH, Alcoba EB. An analysis of reported independent prognostic factors for survival in squamous cell carcinoma of the vulva: is tumor size significance being underrated? Gynecol Oncol. 2014; 132:643–648.
26. Kosary CL. FIGO stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecological system: an analysis of 1973-87 SEER cases of cancers of the endometrium, cervix, ovary, vulva, and vagina. Semin Surg Oncol. 1994; 10:31–46.
27. Hacker NF. Revised FIGO staging for carcinoma of the vulva. Int J Gynaecol Obstet. 2009; 105:105–106.
28. Rouzier R, Preti M, Sideri M, Paniel BJ, Jones RW. A suggested modification to FIGO stage III vulvar cancer. Gynecol Oncol. 2008; 110:83–86.
29. Sznurkowski JJ. Letter to the Editor referring to the manuscript entitled: "New FIGO staging system of vulvar cancer indeed provides a better reflection of prognosis" recently reported by van der Steen S. et al., (Gynecol Oncol 2010;119:520-5. Epub 2010 Sep 28). Gynecol Oncol. 2011; 121:424–425.
30. Moore DH, Ali S, Koh WJ, Michael H, Barnes MN, McCourt CK, et al. A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: a gynecologic oncology group study. Gynecol Oncol. 2012; 124:529–533.
31. Piver MS, Chung WS. Prognostic significance of cervical lesion size and pelvic node metastases in cervical carcinoma. Obstet Gynecol. 1975; 46:507–510.
32. Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990; 38:352–357.
33. Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997; 350:535–540.
34. Perez CA, Grigsby PW, Chao KS, Mutch DG, Lockett MA. Tumor size, irradiation dose, and long-term outcome of carcinoma of uterine cervix. Int J Radiat Oncol Biol Phys. 1998; 41:307–317.
35. Horn LC, Fischer U, Raptis G, Bilek K, Hentschel B. Tumor size is of prognostic value in surgically treated FIGO stage II cervical cancer. Gynecol Oncol. 2007; 107:310–315.
36. International Federation of Gynecology and Obstetrics. Modifications in the staging for stage I vulvar and stage I cervical cancer: report of the FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 1995; 50:215–216.
37. FIGO Committee on Gynecologic Oncology. Guidelines for Gynaecologic cancers staging classifications and clinical practice pages. 3rd ed. FIGO Committee on Gynecologic Oncology;2006. p. 37–62.
38. Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008; 26:5802–5812.
39. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
40. Beller U, Quinn MA, Benedet JL, Creasman WT, Ngan HY, Maisonneuve P, et al. Carcinoma of the vulva. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006; 95:Suppl 1. S7–S27.
41. Magrina JF, Stanhope CR, Weaver AL. Pelvic exenterations: supralevator, infralevator, and with vulvectomy. Gynecol Oncol. 1997; 64:130–135.
42. Chiantera V, Rossi M, De Iaco P, Koehler C, Marnitz S, Fagotti A, et al. Morbidity after pelvic exenteration for gynecological malignancies: a retrospective multicentric study of 230 patients. Int J Gynecol Cancer. 2014; 24:156–164.
43. Chan JK, Sugiyama V, Pham H, Gu M, Rutgers J, Osann K, et al. Margin distance and other clinico-pathologic prognostic factors in vulvar carcinoma: a multivariate analysis. Gynecol Oncol. 2007; 104:636–641.
44. Chan JK, Sugiyama V, Tajalli TR, Pham H, Gu M, Rutgers J, et al. Conservative clitoral preservation surgery in the treatment of vulvar squamous cell carcinoma. Gynecol Oncol. 2004; 95:152–156.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download