Abstract
Purpose
Label adherence for non-vitamin K antagonist oral anticoagulants (NOACs) has not been well evaluated in Asian patients with non-valvular atrial fibrillation (AF). The present study aimed to assess label adherence for NOACs in a Korean AF population and to determine risk factors of off-label prescriptions of NOACs.
Materials and Methods
In this COmparison study of Drugs for symptom control and complication prEvention of AF (CODE-AF) registry, patients with AF who were prescribed NOACs between June 2016 and May 2017 were included. Four NOAC doses were categorized as on- or off-label use according to Korea Food and Drug Regulations.
Results
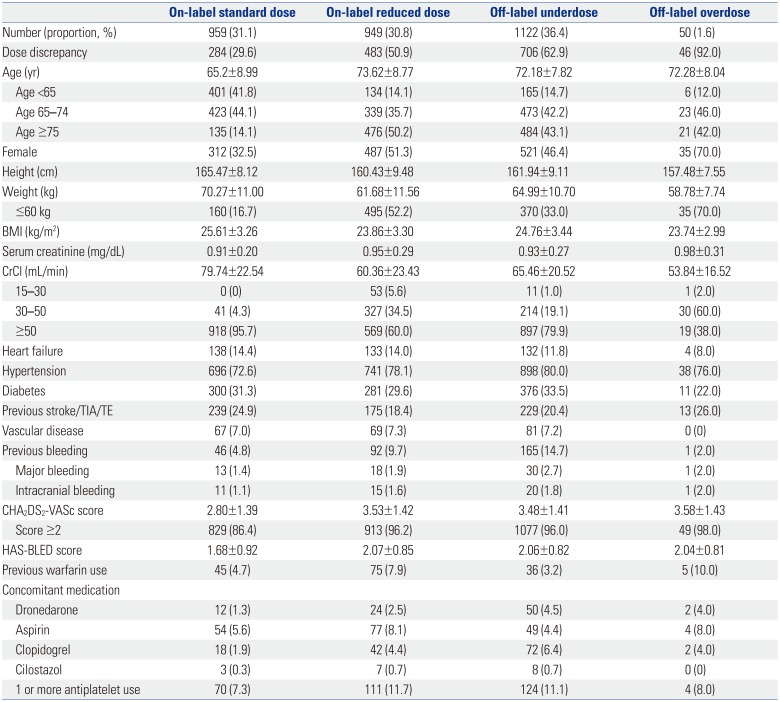
We evaluated 3080 AF patients treated with NOACs (dabigatran 27.2%, rivaroxaban 23.9%, apixaban 36.9%, and edoxaban 12.0%). The mean age was 70.5±9.2 years; 56.0% were men; and the mean CHA2DS2-VASc score was 3.3±1.4. Only one-third of the patients (32.7%) was prescribed a standard dose of NOAC. More than one-third of the study population (n=1122, 36.4%) was prescribed an off-label reduced dose of NOAC. Compared to those with an on-label standard dosing, patients with an off-label reduced dose of NOAC were older (≥75 years), women, and had a lower body weight (≤60 kg), renal dysfunction (creatinine clearance ≤50 mL/min), previous stroke, previous bleeding, hypertension, concomitant dronedarone use, and anti-platelet use.
Adjusted doses of warfarin can be prescribed according to prothrombin time in order to achieve a target therapeutic window, which necessitates frequent monitoring.1 In comparison, non-vitamin K antagonist oral anticoagulants (NOACs) exert a predictable effect without requiring standard anticoagulation monitoring and are suitable alternatives to warfarin for stroke prevention in patients with atrial fibrillation (AF). Their use in clinical practice is increasing rapidly.234 Although the dosage of NOAC is not determined by the results of anticoagulation test results, each NOAC has a recommended dose based on clinical characteristics (e.g., age, body weight, renal function, and concomitant medications). The efficacy and safety of the recommended doses of each NOAC to prevent stroke in patients with AF have been confirmed in pivotal randomized clinical trials.56789 All of these phase 3 trials, except for the RE-LY trial, have provided criteria for dose reduction, which physicians can apply to reduce NOAC doses to decrease the risk of bleeding. In real-world practice, off-label use of a reduced dose of NOAC is not uncommon. In recent reports in the U.S., 13–16% of patients received off-label NOAC doses, and the majority of these patients were prescribed with underdosing.1011 Although reduced doses of NOACs might be considered as an improvement in safety by lowering the risk of bleeding in fragile patients, off-label underdosing of NOACs has been shown to be associated with an increased risk of stroke without a benefit in safety.1011
Compared to Western populations, Asian patients with AF are smaller and have a higher risk of bleeding. Accordingly, physicians prefer to prescribe a reduced dose of NOAC more frequently than physicians in the Western countries.121314 However, there is a paucity of information on whether these reduced doses of NOAC meet the criteria for dose reduction. Therefore, we sought to assess label adherence for NOACs and to identify risk factors associated with off-label prescriptions of NOACs in real-world practice in a Korean population.
The COmparison study of Drugs for symptom control and complication prEvention of AF (CODE-AF) registry is a prospective, multicenter, observational study of AF patients enrolled at 10 tertiary centers in Korea. The study design and centers have been described previously.15 The first database of the CODE-AF registry for analysis was released in May 2017 with patients from June 2016 to April 2017. The data entered at each center were regularly audited, and the database used in this study completed data cleansing. The collected data were registered in the web-based clinical research management system iCReaT (Internet based Clinical Research and Trial management system, http://icreat.nih.go.kr) provided by the Korean government. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki; the study was approved by the ethics committee of each center; and all study patients provided informed consent and were registered at ClinicalTrials.gov (NCT02786095). The study was approved by the Seoul National University Hospital Institutional Review Board (IRB Number: 1605-082-76).
Among the 6275 patients enrolled in the CODE-AF registry, we included patients who were prescribed a NOAC for stroke prevention (n=3213). Patients without available dose information were excluded. Patients without information for evaluation of compliance with labeled dosing, such as age, sex, body weight, or serum creatinine value, were also excluded. We also excluded patients with end-stage renal disease who were on dialysis.
A standard dose was defined, according to each NOAC, as dabigatran 150 mg twice daily, rivaroxaban 20 mg once daily, apixaban 5 mg twice daily, and edoxaban 60 mg once daily. Approved dose reduction criteria were specific to each NOAC according to the following patient characteristics: baseline age, body weight, and serum creatinine level at enrollment. Creatinine clearance (CrCl) was calculated using the Cockcroft-Gault equation.
In the present study, adherence with labeled dosing for each NOAC in each study patient was evaluated based on the Ministry of Food and Drug Safety labeling (Supplementary Table 1, only online).
Patients were categorized into four groups based on NOAC dose and dose recommendation adherence: on-label standard dose, on-label reduced dose, underdosed, and overdosed. To identify predictors of those who had an underdosed prescription, we compared the clinical characteristics between patients who were prescribed an on-label standard dose and those with an underdose who needed to be prescribed the standard dose.
Exclusively for dabigatran, both the 150 mg and 110 mg twice daily doses were regarded as on-label use, because dabigatran does not have mandatory dose reduction criteria for selected patients.59 Therefore, dabigatran 110 mg twice daily prescription was defined as the on-label reduced dose, regardless of patients' baseline characteristics. Instead, the Ministry of Food and Drug Safety labeling recommended the use of dabigatran 110 mg bid in those with CrCl 30–50 mL/min or age ≥75 years. Therefore, for patients prescribed with a reduced dose of dabigatran, we defined the patients who met one of these criteria as “recommendation-concordant prescription,” and those who did not meet any of these criteria as “recommendation-discordant prescription.”16
Differences in dose reduction criteria for each NOAC could result in a discrepancy in prescriptions with standard or reduced doses among the NOACs. For example, one NOAC should be prescribed with a standard dose, whereas another NOAC could be prescribed with a reduced dose. To evaluate this discrepancy in dose reductions among NOACs, we applied dose reduction criteria to each NOAC of the total population, evaluated the dose reduction criteria of the four NOACs in each patient, and determined which NOAC could be prescribed as a labeled reduced dose, whereas other NOACs could not.
Continuous variables are presented as means and standard deviations. Comparison of continuous variables was performed using an independent t-test or, in case of a non-normal distribution, the Mann-Whitney test. Categorical variables are presented as numbers and percentages and were compared using the chi-square test of Fisher's exact test. To assess factors associated with the prescription of off-label reduced doses of NOACs, univariate binary logistic regression analysis was used. All variables with p<0.1 in the univariate analysis were used for multivariate logistic regression analysis. The CHA2DS2-VASc and HAS-BLED scores were excluded in the multivariable logistic regression because of interactions with variables included in the clinical scores. Sensitivity analysis was performed including CHA2DS2-VASc and HAS-BLED scores. Odds ratios were calculated as an estimate of the risk associated with a particular variable with a 95% confidence interval based on binomial distributions. All statistical analyses were performed with SPSS statistical package version 19.0 (IBM Corp., Armonk, NY, USA). All two-tailed p values <0.05 were considered statistically significant.
We evaluated 3080 AF patients treated with NOACs (dabigatran 27.2%, rivaroxaban 23.9%, apixaban 36.9%, and edoxaban 12.0%). The baseline characteristics are presented in Supplementary Table 2 (only online). The mean age was 70.5±9.2 years; 56.0% were men; 34% were under 60 kg; and the mean CHA2DS2-VASc score was 3.3±1.4 (CHA2DS2-VASc score ≥2, 93.1%). Six hundred seventy-seven (22%) patients had moderate renal dysfunction (CrCl <50 mL/min). Eighty-eight (2.9%) patients were prescribed dronedarone concomitantly. Among the NOAC groups, the apixaban group was older and had a higher CHA2DS2-VASc score than the other NOAC groups.
In the total study population, 32.7% of patients were prescribed a standard dose, and 67.3% of patients received a reduced dose. There was a difference in the prescription patterns of dose reduction by each NOAC (71.8% in dabigatran, 69.3% in rivaroxaban, 66.3% in apixaban and 56.6% in edoxaban). Patients taking dabigatran and rivaroxaban were prescribed a reduced dose more frequently than those taking apixaban and edoxaban, whereas edoxaban was prescribed as a reduced dose in only half of patients (all, p<0.05, Supplementary Table 2, only online).
Among the entire study population, on-label users accounted for 61.9% (31.1% with a standard dose and 30.8% with a reduced dose). Those who were off-label underdosed comprised 36.4%, and off-label overdosed included 1.6% (Table 1, Fig. 1). Most patients taking dabigatran were prescribed labeled used, although there were a few patients with off-label use (once daily prescription or overdose prescription in 16 cases). Edoxaban showed the highest dose recommendation adherence (on-labeled use, 68%), whereas rivaroxaban showed the lowest dose recommendation adherence (on-labeled use, 43.9%). Among those with a standard dose (n=1009), most patients (95%) adhered with the labeling (Fig. 2). Most patients with a standard dose of dabigatran, rivaroxaban, and apixaban followed on-label prescription, while edoxaban showed a higher proportion of overdosed prescriptions, compared to other NOACs (0, 7, 0 and 20%, respectively, p<00.001) (Fig. 2). The reasons for this overdose prescription of edoxaban included patients being underweight (≤60 kg, n=18), moderate-to-severe renal dysfunction (CrCl 15–50 mL/min, n=6), or both (n=8). Among those taking a reduced dose (n=2071), only 46.3% of patients had on-label use, whereas more than half of patients (53.7%) had off-label use.
The proportions of off-label underdosed prescription differed among NOACs. Rivaroxaban and apixaban showed similar, but higher, prescriptions of off-label reduced doses (78% and 83%, respectively, p=0.051) than edoxaban, whereas the edoxaban group showed only 41% of off-label reduced dose prescription (rivaroxaban vs. edoxaban, apixaban vs. edoxaban, both p<0.001). The reasons for off-label use in each NOAC were as follows: Among 396 patients taking underdosed rivaroxaban, 320 (81%) patients had normal renal function, and 76 (19%) patients were prescribed a dose less than 10 mg. Among 624 patients with underdosed apixaban, 300 (48%) patients met one dose reduction criterion; 274 (44%) patients met no dose reduction criteria; and 50 (8%) patients were prescribed a once-daily dosage or less than 2.5 mg bid. Among 87 patients with underdosed edoxaban, 79 (91%) patients met none of the dose reduction criteria, and 8 patients were prescribed a 15 mg dosage. Among the patients with a reduced dose of dabigatran, 40.2% received a recommendation-concordant reduced dose, and 59.8% were prescribed a recommendation-discordant reduced dose. The reasons for recommendation-discordant use in dabigatran included normal renal function (above CrCl 50 mL/min, n=480) and age younger than 75 years (n=380). We summarized label adherence for each NOAC in Supplementary Fig. 1 (only online). Apixaban showed the smallest number of patients who met the dose reduction criteria (12.9%), whereas edoxaban showed the largest number of patients who met the dose reduction criteria (43.4%). Compared to real-world prescriptions, edoxaban showed a smaller discrepancy between dose label and prescription, compared to rivaroxaban and apixaban (rivaroxaban vs. edoxaban, apixaban vs. edoxaban, both p<0.001).
The baseline characteristics by NOAC dosing are presented in Table 1. To identify factors associated with off-label underdosed NOACs, we compared the baseline characteristics of patients prescribed an off-label underdosed NOAC to patients prescribed an on-label standard dose (Supplementary Table 3, only online). Relative to the on-label standard dose group, patients who received off-label underdosed NOACs were significantly older, more likely women, had a lower body weight, had renal impairment and were more likely to have hypertension (all, p<0.001). Compared to those with on-label standard dose NOACs, the off-label underdosed group showed significantly higher CHA2DS2-VASc scores (mean 3.48±1.41 vs. 2.80±1.39) and HAS-BLED scores (mean 2.06±0.82 vs. 1.68±0.92; all, p<0.001). Previous bleeding was more common in the off-label underdosed group than the on-label standard dose group (14.7% vs. 4.8%, p<0.001), whereas previous thromboembolic events (TE) were more common in the on-label standard dose group compared to the off-label underdosed group (24.9% vs. 20.4%, p=0.014). Rates of one or more concomitant antiplatelet use were significantly higher in the off-label underdosed group than the on-label standard dose group (11.1% vs. 7.3%, p=0.003). Dronedarone use was more common in the off-label underdosed group compared to the on-label standard dose group (4.5% vs. 1.3%, p<0.001). In the multivariable logistic regression, old age (≥75 years), women, a lower body weight (≤60 kg), renal impairment (CrCl ≤50 mL/min), prevalent hypertension, previous stroke/transient ischemic attack (TIA)/TE, previous bleeding, dronedarone use, and anti-platelet use were associated with off-label underdosing (Fig. 3). These findings were similar when the CHA2DS2-VASc and HASBLED scores were included in multivariable analysis (Supplementary Table 4, only online).
Each NOAC has different dose reduction criteria and the proportions of off-label underdosed prescription were different among NOACs. We evaluated the associated factors with off-label underdosed prescription in each NOAC group (Supplementary Tables 5, 6, 7, 8, Supplementary Fig. 2, only online). Although the factors varied across the types of NOACs due to patient numbers of each group, the associated factors with offlabel underdosing were largely consistent with the results from total study population (Fig. 3).
In this study, we analyzed label adherence for dosing across four NOACs and factors associated with off-label underdosing in patients with AF in routine clinical practice. In this large-scale Asian AF cohort who were prescribed NOACs, the main findings were as follows: 1) off-label NOAC prescription was frequently observed, and label adherence for NOAC dosing was about 60%; 2) most standard doses of NOAC were prescribed as in label, although more than half of the reduced doses of NOAC were prescribed off-label; 3) edoxaban showed the highest dose recommendation adherence, whereas rivaroxaban showed the lowest dose recommendation adherence; and 4) old age (≥75 years), women, lower body weight (≤60 kg), renal impairment (CrCl ≤50 mL/min), prevalent hypertension, previous stroke/TIA/TE, previous bleeding, dronedarone use, and anti-platelet use were associated with off-label underdosing prescriptions of NOACs. These findings could give important insights into real-world NOAC prescription behavior in Asian populations.
Patients taking NOACs have favorable risk-benefit profiles, few drug interactions, and require less intensive monitoring than those taking warfarin. Therefore, NOACs are being prescribed more frequently for stroke prevention in nonvalvular AF.23417 Each NOAC requires a different dose reduction strategy as described in their phase 3 clinical trials (Supplementary Table 1, only online). The proportion of patients who indicated dose reductions were different among pivotal clinical trials (Supplementary Fig. 3, only online).5678 Dabigatran did not have dose reduction criteria in RE-LY, and patients were randomly assigned doses of 150 mg or 110 mg twice daily.5 In the ROCKET AF trials, 21% of the rivaroxaban group who had moderate renal impairment (CrCl 30–49 mL/min) received rivaroxaban 15 mg once daily.6 In the ARISTOTLE trials, less than 5% of the study population was recommended for dose reduction.7 In the high-dose regimen edoxaban group in the ENGAGE AFTIMI 48 trial, 25% of the patients indicated a dose reduction.8 We applied dose reduction criteria for each NOAC to the total population to calculate the proportion of patients who were indicated for a dose reduction (Supplementary Fig. 4, only online). Apixaban permitted the smallest proportion of the population (9.6%) to use a reduced dose by their drug label, whereas edoxaban permitted the largest proportion of the population (44%). The proportion of indicated dose reductions was quite similar in cases of rivaroxaban, compared to clinical trials (22% vs. 21%). For apixaban and edoxaban, more patients were indicated to have a reduced dose of NOACs in the real-world population, compared with clinical trials (apixaban, 9.6% vs. 4.7%; and edoxaban 44% vs. 25.4%). These findings might be caused by a lower body weight in Asian populations. Interestingly, the agreement of dose reductions among all four NOAC drug labels was observed only in 5.6% of the total study population. Forty-three percent of the total population was recommended a standard dose of NOAC. However, in 49.3% of patients, a discrepancy in NOAC dosing was observed. Therefore, half of patients might be prescribed either a standard dose or reduced dose with different NOACs, not violating the dose reduction label.
The appropriate use of NOACs is an emerging issue. There are several reports regarding the labeled use of NOAC in patients with AF. Recently, in the ORBIT-AF II (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II) registry, 12.8% of the total study population received NOACs inconsistent with the drug label (9.4% with an off-label underdose and 3.4% with an off-label overdose).10 In a large recent U.S. claims database, 83.7% of the total patients were prescribed the recommended dose of NOACs, while 12.0% and 4.3% of the study patients received off-label underdose and overdose NOACs, respectively.11 Although reduced dose NOACs are commonly used in Asian patients with AF, there are no data about “label adhered dosing” in the Asian population. According to Taiwanese nationwide data, almost 90% of the total study population was prescribed a reduced dose of NOACs (110 mg twice daily dabigatran and 10 mg to 15 mg once daily rivaroxaban).12 Additionally, in the Korean nationwide data, 40–60% of the patients were prescribed a reduced dose of NOACs.14 However, neither studies reported label adherence for NOAC dosing. In our study, 67.3% of patients were prescribed a reduced dose of NOACs, regardless of label adherence, and 38% of the total population did not meet the recommended drug labeling use. Most off-label dosing was considered as off-label underdose NOAC use (96% of off-label dosing population). Namely, patients who were a recommended standard dose of NOACs, based on their baseline characteristics, frequently received a reduced dose of NOACs.
There are several explanations regarding the reason for the preference of reduced doses of NOAC prescriptions in the Asian population. First, the Asian population has a lower body mass, and some clinicians who targeted a low international normalized ratio in older adult patients replaced warfarin with a reduced dose of NOAC.12 Furthermore, different approved doses were used in some Asian countries based on clinical evidence. In Japan, 15 mg rivaroxaban was recommended as a standard dose instead of 20 mg, based on the J-ROCKET-AF study.18 Second, the Asian population tends to have a higher risk of intracranial hemorrhage (ICH) than the Western population, for which clinicians feel it important not to increase the risk of bleeding.13 Based on these results, we could assume that physicians tend to choose reduced dose NOACs for patients regarded as more fragile to bleeding. In addition, when patients are in the “borderline gray zone” by label, physicians were more likely to select a reduced dose NOAC. Compared to the on-label standard dose group, patients with an off-label underdose of NOACs were more likely to have a dosing discrepancy based on the drug-specific label (62.9% vs. 29.6%, respectively; p<0.001).
A few previous reports have outlined the clinical consequences of label adherence. In the ENGAGE AF-TIMI 48 trial, a low-dose edoxaban regimen posed a significantly higher ischemic stroke risk, whereas patients with the same dose of edoxaban who met one of the dose reduction criteria showed a comparable risk of stroke to those taking warfarin.8 Recently, adverse clinical consequences for off-label dosing have been reported from real-world data. In the ORBIT-AF II registry, compared to the recommended dose of NOACs, off-label doses of NOACs showed higher adverse events rates.10 Particularly, an offlabel underdose of NOAC was associated with a worsened effectiveness with no benefit in safety.1011 Notably, the estimated stroke risk was significantly higher in the off-label underdose group, compared to the on-label standard dose group. To improve the clinical outcomes of Asian AF patients, an increase in label adherence for NOAC dosing might be needed.
There are a few reports suggesting that lower dose NOACs regarded as “off-label underdose” by current labels could be safe and effective in the Asian population. Although 15 mg rivaroxaban was recommended as a standard dose in patients without renal impairment in the J-ROCEKT-AF trial, the number of patients was small to generalize for Asian patients.18 However, although dabigatran showed different positions in terms of label adherence, recently, patients prescribed recommendation-discordant 110 mg dabigatran present comparable results in effectiveness and safety, compared to patients with 150 mg dabigatran, among Korean AF patients.16 Based on the Taiwanese nationwide cohort, universally-prescribed reduced doses of dabigatran and rivaroxaban, regardless of label adherence, showed better effectiveness and safety, compared to the real-world warfarin group.12 Considering that Asians have a lower body mass, more prevalent ICH, and generally a lower time in the therapeutic range, further investigation is needed to assess the optimal doses of NOACs and net clinical effects of off-label NOAC prescription in the Asian AF population.12131920
There are several limitations in the present study. We analyzed NOAC dosing patterns based on patients and their baseline characteristics. However, body weight, renal function, and concomitant drug use could be modified during follow-up and might affect the physician's choice of NOAC dosing. In addition, in this study, we did not include follow-up data; therefore, clinical outcomes according to inappropriate NOAC dosing were not evaluated. Further investigation will be needed to determine the clinical implications of NOAC dosing in the Korean AF population. Lastly, this study analyzed the multicenter prospective registry of Korean population. Thus, ethnic uniformity should be considered, and the generalization of the study results should be proceeded with caution. Moreover, among East Asian countries, definitions of “on-label dose” for rivaroxaban differ, as do proportions of low dose prescription of NOACs. Despite these limitations, this study describes a prospectively-collected large number of Korean AF patients. Our findings reflect the real-world clinical practice patterns of NOAC dosing in contemporary Asian patients with AF.
In conclusion, in real-world practice, half of Korea patients treated with NOACs received off-label reduced dose. Older age, female sex, lower body weight, impaired renal function, previous stroke or bleeding history, hypertension, and concomitant dronedarone or antiplatelet agent use were independently associated with off-label underdose prescription. Further study is needed to investigate the clinical outcomes of off-label prescription of NOACs, especially in Asians.
ACKNOWLEDGEMENTS
This study was supported by the Korea National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2014R1A1A2A16055218), Korea Government (Ministry of Science, ICT and Future Planning) (NRF-2015R1C1A2A01054767), the Korean Healthcare Technology R&D project funded by the Ministry of Health and Welfare (HI15C1200), and also by the Technology Innovation Program or Industrial Strategic Technology Development Program (10052668, development of wearable self-powered energy source and low-power wireless communication system for a pacemaker) funded by the Ministry of Trade, Industry and Energy (MOTIE, Sejong, Korea).
Notes
AUTHOR CONTRIBUTIONS:
Conceptualization: Choi EK.
Data curation: Joung B, Lee SR.
Formal analysis: Lee SR, Park JS.
Funding acquisition: Joung B, Choi EK.
Investigation: Lee SR, Cha MJ.
Methodology: Kim TH, Park J, Park JK.
Project administration: Lee JM, Kang KW, Shim J.
Resources: Uhm JS, Kim J.
Software: Kim C, Kim JB.
Supervision: Park HW.
Validation: Park JS.
Visualization: Lee SR.
Writing—original draft: Lee SR.
Writing—review & editing: Joung B, Choi EK, Lee Y.
References
1. Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010; 123:638–645. PMID: 20609686.

2. Huisman MV, Rothman KJ, Paquette M, Teutsch C, Diener HC, Dubner SJ, et al. The changing landscape for stroke prevention in AF: findings from the GLORIA-AF registry phase 2. J Am Coll Cardiol. 2017; 69:777–785. PMID: 28209218.
3. Camm AJ, Accetta G, Ambrosio G, Atar D, Bassand JP, Berge E, et al. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart. 2017; 103:307–314. PMID: 27647168.

4. Lee SR, Choi EK, Han KD, Cha MJ, Oh S, Lip GYH. Temporal trends of antithrombotic therapy for stroke prevention in Korean patients with non-valvular atrial fibrillation in the era of non-vitamin K antagonist oral anticoagulants: a nationwide population-based study. PLoS One. 2017; 12:e0189495. PMID: 29261716.

5. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139–1151. PMID: 19717844.

6. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365:883–891. PMID: 21830957.

7. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365:981–992. PMID: 21870978.
8. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013; 369:2093–2104. PMID: 24251359.

9. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016; 37:2893–2962. PMID: 27567408.

10. Steinberg BA, Shrader P, Thomas L, Ansell J, Fonarow GC, Gersh BJ, et al. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II Registry. J Am Coll Cardiol. 2016; 68:2597–2604. PMID: 27978942.
11. Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. 2017; 69:2779–2790. PMID: 28595692.

12. Chan YH, Kuo CT, Yeh YH, Chang SH, Wu LS, Lee HF, et al. Thromboembolic, bleeding, and mortality risks of rivaroxaban and dabigatran in Asians with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2016; 68:1389–1401. PMID: 27659460.
13. Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007; 50:309–315. PMID: 17659197.

14. Cha MJ, Choi EK, Han KD, Lee SR, Lim WH, Oh S, et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in Asian patients with atrial fibrillation. Stroke. 2017; 48:3040–3048. PMID: 28974629.

15. Kim H, Kim TH, Cha MJ, Lee JM, Park J, Park JK, et al. A prospective survey of atrial fibrillation management for real-world guideline adherence: COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF) registry. Korean Circ J. 2017; 47:877–887. PMID: 29171211.

16. Lee KH, Park HW, Lee N, Hyun DY, Won J, Oh SS, et al. Optimal dose of dabigatran for the prevention of thromboembolism with minimal bleeding risk in Korean patients with atrial fibrillation. Europace. 2017; 19(suppl_4):iv1–iv9. PMID: 29220421.

17. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014; 383:955–962. PMID: 24315724.

18. Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation - the J-ROCKET AF study. Circ J. 2012; 76:2104–2111. PMID: 22664783.
19. Oh S, Goto S, Accetta G, Angchaisuksiri P, Camm AJ, Cools F, et al. Vitamin K antagonist control in patients with atrial fibrillation in Asia compared with other regions of the world: real-world data from the GARFIELD-AF registry. Int J Cardiol. 2016; 223:543–547. PMID: 27552578.

20. Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014; 111:789–797. PMID: 24500243.

SUPPLEMENTARY MATERIALS
Supplementary Table 3
Comparison of Baseline Characteristics between On-Label Standard Dose and Off-Label Underdose Groups
Supplementary Table 4
Multivariable Logistic Regression for Factors Associated with Off-Label Underdosing, Including CHA2DS2-VASc and HAS-BLED Score
Supplementary Table 5
Comparison of Baseline Characteristics between On-Label Standard Dose and Off-Label Underdose Groups in Patients Treated with Dabigatran
Supplementary Table 6
Comparison of Baseline Characteristics between On-Label Standard Dose and Off-Label Underdose Groups in Patients Treated with Rivaroxaban
Supplementary Table 7
Comparison of Baseline Characteristics between On-Label Standard Dose and Off-Label Underdose Groups in Patients Treated with Apixaban
Supplementary Table 8
Comparison of Baseline Characteristics between On-Label Standard Dose and Off-Label Underdose Groups in Paitents Treated with Edoxaban
Supplementary Fig. 1
Comparison of dose recommendations according to drug-specific label and real-world NOAC dosing for each NOAC. NOAC, non-vitamin K antagonist oral anticoagulant.
Supplementary Fig. 2
Factors associated with off-label underdosing according to each NOAC. NOAC, non-vitamin K antagonist oral anticoagulants; bwt, body weight; CrCl, creatinine clearance; TE, thromboembolic events; TIA, transient ischemic attack; CI, confidence interval.
Supplementary Fig. 3
Dose proportions in pivotal RCTs for four NOACs. NOAC, non-vitamin K antagonist oral anticoagulant; RCT, randomized clinical trial; HDER, high dose edoxaban regimen (edoxaban 60/30 mg). *Dabigatran did not have specific dose reduction criteria for selected patients between the 150 mg and 110 mg twice daily doses in the RE-LY trial.
Supplementary Fig. 4
Dose proportions in the total study population according to each drug's label. NOAC, non-vitamin K antagonist oral anticoagulant.
Fig. 1
Differences in drug-specific dose reduction recommendations for each NOAC and discrepancies in NOAC dosing. NOAC, non-vitamin K antagonist oral anticoagulant.
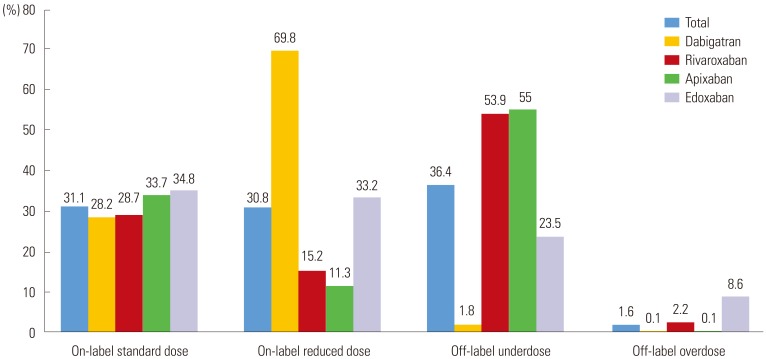
Fig. 2
Label adherence for NOAC dosing in the study population. NOAC, non-vitamin K antagonist oral anticoagulant.
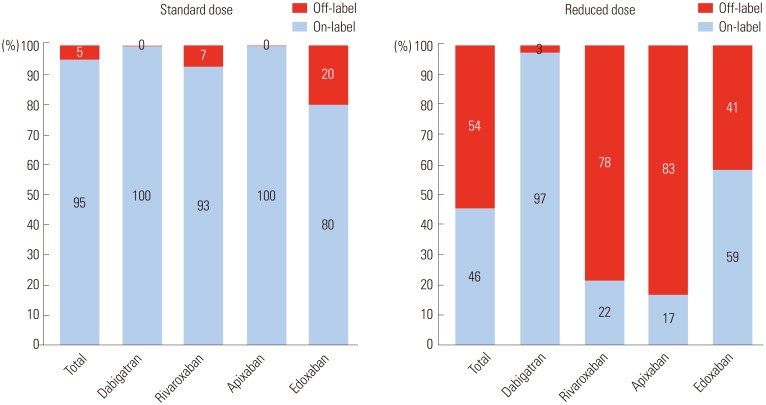
Fig. 3
Factors associated with off-label underdosing. CrCl, creatinine clearance; TE, thromboembolic events; TIA, transient ischemic attack; CI, confidence interval.
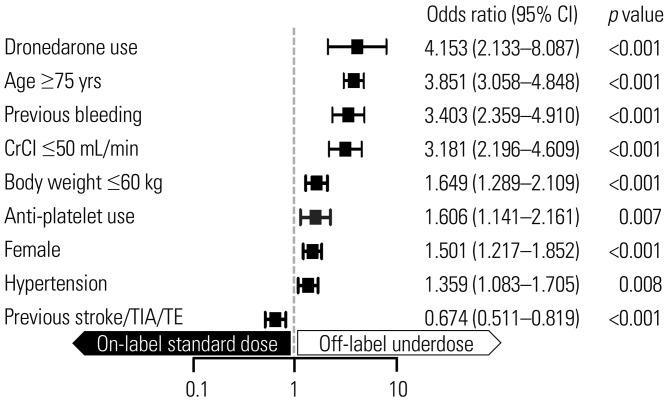
Table 1
Baseline Characteristics according to Labeled Use




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download