1. Kim D, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, et al. 10-year nationwide trends of the incidence, prevalence, and adverse outcomes of non-valvular atrial fibrillation nationwide health insurance data covering the entire Korean population. Am Heart J. 2018; 202:20–26. PMID:
29802976.

2. Kim D, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, et al. Increasing trends in hospital care burden of atrial fibrillation in Korea, 2006 through 2015. Heart. 2018; 104:2010–2017. PMID:
29666179.

3. Kim TH, Yang PS, Uhm JS, Kim JY, Pak HN, Lee MH, et al. CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥75 [doubled], diabetes mellitus, prior stroke or transient ischemic attack [doubled], vascular disease, age 65–74, female) for stroke in Asian patients with atrial fibrillation: a Korean nation-wide sample cohort study. Stroke. 2017; 48:1524–1530. PMID:
28455320.
4. Yang PS, Ryu S, Kim D, Jang E, Yu HT, Kim TH, et al. Variations of prevalence and incidence of atrial fibrillation and oral anticoagulation rate according to different analysis approaches. Sci Rep. 2018; 8:6856. PMID:
29717165.

5. O'Brien EC, Holmes DN, Thomas LE, Fonarow GC, Allen LA, Gersh BJ, et al. Prognostic significance of nuisance bleeding in anticoagulated patients with atrial fibrillation. Circulation. 2018; 138:889–897. PMID:
29678813.
6. Kiernan DF, Hariprasad SM, Rusu IM, Mehta SV, Mieler WF, Jager RD. Epidemiology of the association between anticoagulants and intraocular hemorrhage in patients with neovascular age-related macular degeneration. Retina. 2010; 30:1573–1578. PMID:
21060269.

7. Witmer MT, Cohen SM. Oral anticoagulation and the risk of vitreous hemorrhage and retinal tears in eyes with acute posterior vitreous detachment. Retina. 2013; 33:621–626. PMID:
23108264.

8. Biyik I, Mercan I, Ergene O, Oto O. Ocular bleeding related to warfarin anticoagulation in patients with mechanical heart valve and atrial fibrillation. J Int Med Res. 2007; 35:143–149. PMID:
17408066.

9. Brown JS, Mahmoud TH. Anticoagulation and clinically significant postoperative vitreous hemorrhage in diabetic vitrectomy. Retina. 2011; 31:1983–1987. PMID:
21836531.

10. Olson JM, Scott IU, Kerchner DL, Kunselman AR. Association between systemic anticoagulation and rate of intraocular hemorrhage following intravitreal anti-VEGF therapy for age-related macular degeneration. Ophthalmic Surg Lasers Imaging Retina. 2013; 44:455–459. PMID:
24044707.

11. Benzimra JD, Johnston RL, Jaycock P, Galloway PH, Lambert G, Chung AK, et al. The Cataract National Dataset electronic multicentre audit of 55,567 operations: antiplatelet and anticoagulant medications. Eye (Lond). 2009; 23:10–16. PMID:
18259210.

12. Katz J, Feldman MA, Bass EB, Lubomski LH, Tielsch JM, Petty BG, et al. Risks and benefits of anticoagulant and antiplatelet medication use before cataract surgery. Ophthalmology. 2003; 110:1784–1788. PMID:
13129878.

13. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017; 46:e15. PMID:
26822938.

14. Lee H, Kim TH, Baek YS, Uhm JS, Pak HN, Lee MH, et al. The trends of atrial fibrillation-related hospital visit and cost, treatment pattern and mortality in Korea: 10-year nationwide sample cohort data. Korean Circ J. 2017; 47:56–64. PMID:
28154592.

15. Baek YS, Yang PS, Kim TH, Uhm JS, Park J, Pak HN, et al. Associations of abdominal obesity and new-onset atrial fibrillation in the general population. J Am Heart Assoc. 2017; 6:e004705. PMID:
28588091.

16. Lee HY, Yang PS, Kim TH, Uhm JS, Pak HN, Lee MH, et al. Atrial fibrillation and the risk of myocardial infarction: a nation-wide propensity-matched study. Sci Rep. 2017; 7:12716. PMID:
28983076.

17. Song S, Yang PS, Kim TH, Uhm JS, Pak HN, Lee MH, et al. Relation of chronic obstructive pulmonary disease to cardiovascular disease in the general population. Am J Cardiol. 2017; 120:1399–1404. PMID:
28826898.

18. Sahai H, Khurshid A. Statistics in epidemiology: methods, techniques, and applications. Boca Raton (FL): CRC Press;1996.
19. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007; 146:857–867. PMID:
17577005.

20. Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010; 123:638–645. PMID:
20609686.

21. Kim H, Kim TH, Cha MJ, Lee JM, Park J, Park JK, et al. A prospective survey of atrial fibrillation management for real-world guideline adherence: COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF) Registry. Korean Circ J. 2017; 47:877–887. PMID:
29171211.

22. O'Brien EC, Simon DN, Allen LA, Singer DE, Fonarow GC, Kowey PR, et al. Reasons for warfarin discontinuation in the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF). Am Heart J. 2014; 168:487–494. PMID:
25262258.
23. Gumbinger C, Holstein T, Stock C, Rizos T, Horstmann S, Veltkamp R. Reasons underlying non-adherence to and discontinuation of anticoagulation in secondary stroke prevention among patients with atrial fibrillation. Eur Neurol. 2015; 73:184–191. PMID:
25633474.

24. Altman R, Rivas AJ, Gonzalez CD. Bleeding tendency in dual antiplatelet therapy with aspirin/clopidogrel: rescue of the template bleeding time in a single-center prospective study. Thromb J. 2012; 10:3. PMID:
22236361.

25. Rosenman MB, Baker L, Jing Y, Makenbaeva D, Meissner B, Simon TA, et al. Why is warfarin underused for stroke prevention in atrial fibrillation? A detailed review of electronic medical records. Curr Med Res Opin. 2012; 28:1407–1414. PMID:
22746356.

26. Lindgren G, Sjödell L, Lindblom B. A prospective study of dense spontaneous vitreous hemorrhage. Am J Ophthalmol. 1995; 119:458–465. PMID:
7709970.

27. Oh J, Smiddy WE, Kim SS. Antiplatelet and anticoagulation therapy in vitreoretinal surgery. Am J Ophthalmol. 2011; 151:934–939. PMID:
21411057.

28. Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013; 381:1107–1115. PMID:
23415013.

29. Rubboli A, Faxon DP, Juhani Airaksinen KE, Schlitt A, Marín F, Bhatt DL, et al. The optimal management of patients on oral anticoagulation undergoing coronary artery stenting. The 10th anniversary overview. Thromb Haemost. 2014; 112:1080–1087. PMID:
25298351.
30. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017; 377:1513–1524. PMID:
28844193.

31. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016; 375:2423–2434. PMID:
27959713.

32. Golwala HB, Cannon CP, Steg PG, Doros G, Qamar A, Ellis SG, et al. Safety and efficacy of dual vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of randomized clinical trials. Eur Heart J. 2018; 39:1726–1735a. PMID:
29668889.

33. Kuhli-Hattenbach C, Fischer IB, Schalnus R, Hattenbach LO. Subretinal hemorrhages associated with age-related macular degeneration in patients receiving anticoagulation or antiplatelet therapy. Am J Ophthalmol. 2010; 149:316–321. PMID:
19939348.

34. Ying GS, Maguire MG, Daniel E, Grunwald JE, Ahmed O, Martin DF. Comparison of Age-Related Macular Degeneration Treatments Trials Research Group. Association between antiplatelet or anticoagulant drugs and retinal or subretinal hemorrhage in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016; 123:352–360. PMID:
26545320.
35. Moon SW, Oh J, Yu HG, Cho HY, Song SJ. Incidence and risk factors for macular hemorrhage following intravitreal ranibizumab injection for neovascular age-related macular degeneration. J Ocul Pharmacol Ther. 2013; 29:556–559. PMID:
23480269.

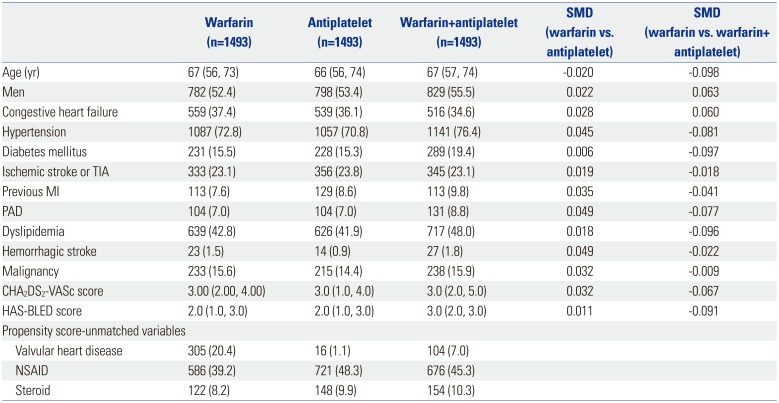
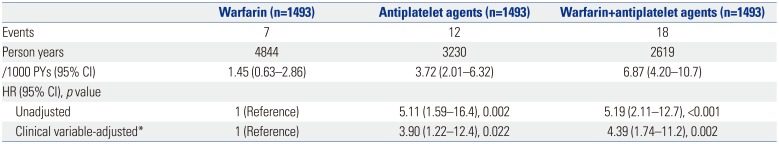
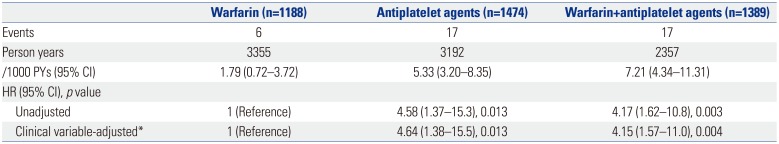




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download