Abstract
Dendritic cells (DCs) play a key role not only in the initiation of primary immune responses, but also in the development and maintenance of immune tolerance. Numerous protocols have been developed to generate tolerogenic DCs (tolDCs) ex vivo, and the therapeutic efficacy of ex vivo-generated tolDCs has been demonstrated in autoimmune disease animal models. Based on successes in small animal models, several clinical trials have been completed or are on-going in patients with autoimmune diseases such as rheumatoid arthritis, type 1 diabetes, multiple sclerosis, and Crohn's disease. Here we describe the methods used to generate tolDCs ex vivo, and the common features shared by tolDCs. In addition, we overview five completed clinical trials with reported outcomes and summarize the tolDC-based clinical trials that are currently registered with the U.S. National Institutes of Health. Although the number of tolDC-based clinical trials is much smaller than the hundreds of clinical trials using immunogenic DCs, tolDC-based treatment of autoimmune diseases is becoming a reality, and could serve as an innovative cellular therapy in the future.
Dendritic cells (DCs) are the most potent antigen presenting cells, which are crucial for the induction of T cell responses.12 DCs can acquire and process antigens in the periphery, and migrate to secondary lymphoid tissues where they prime primary T cell responses. While DCs play a key role in the initiation of primary immune responses, they also play a crucial role in the development and maintenance of immune tolerance.345
The functional difference between immunogenic and tolerogenic DCs depends on maturation state and maturation environment. Immature tissue-resident DCs sense invading antigens via pattern-recognition receptors such as toll-like receptors, take up the antigens via phagocytosis or endocytosis and degrade them into small peptides to present the antigenic peptides in association with major histocompatibility complex (MHC) class II molecules to CD4 T cells.1 These antigen entrapping and processing processes trigger the maturation of immature DCs to mature DCs, which express considerably higher levels of co-stimulatory molecules such as CD80 and CD86, and MHC class II molecules, and secrete considerably higher amounts of proinflammatory cytokines, such as interleukin (IL)-1, IL-6, IL-12, and tumor necrosis factor (TNF)-α, compared to immature DCs.6
Tissue-resident steady-state DCs are immature DCs, which express low levels of co-stimulatory molecules and moderate levels of MHC class II molecules, and are poorly immunogenic unless activated. In fact, steady-state immature DCs, which display peptides originating from self-proteins in association with MHC molecules on the cell surface, are tolerogenic DCs (tolDCs) that maintain self-tolerance against self-antigens.7 A number of attempts have been made to use tolerogenic immature DCs to induce immune tolerance. Dhodapkar and Steinman generated immature DCs using IL-4 and granulocyte macrophage-colony stimulating factor (GM-CSF), pulsed them with antigen, and then injected them into humans. They showed that injection of antigen-pulsed immature DCs led to antigen-specific inhibition of effector T cell function by inducing regulatory T cells (Tregs).89 However, using tolerogenic immature DCs to induce immune tolerance raises concerns regarding the functional stability of the immature state, because immature DCs could be converted into immunogenic mature DCs when encountering a ‘danger signal’ such as proinflammatory cytokines and microbial products. Thus, one of the major challenges facing tolDC-based immunotherapy is optimizing the protocol for obtaining functionally stable tolDCs.
TolDCs with durable immaturity and immune regulatory properties have been generated ex vivo using various pharmacological agents such as rapamycin, dexamethasone, and vitamin D.10 Immunosuppressive cytokines such as IL-10 and transforming growth factor (TGF)-β have also been used to induce tolDCs.11 In general, tolDCs are characterized by reduced expression of co-stimulatory molecules and IL-12, decreased ability to induce T cell proliferation, increased IL-10 secretion, and increased Treg induction.1011 The mechanisms underlying tolDC activity include the induction of Tregs, increasing the expression of programmed death-ligand 1 (PD-L1) and inducible costimulator ligand (ICOSL), and the production of immunosuppressive factors such as IL-10 and TGF-β.711121314
Antigen-pulsed tolDCs are promising tools for generating antigen-specific immune tolerance. They can be infused directly for the induction of antigen-specific immune tolerance in vivo, or can be used to generate antigen-specific Tregs in vitro for Treg-based adaptive cell therapy. In this review, we describe the methods used to generate tolDCs ex vivo and the phenotypic and functional characteristics of the induced tolDCs. In addition, we discuss the therapeutic potential of tolDCs for treating immune disorders based on completed or currently on-going clinical trials with tolDCs.
Human tolDCs are mostly produced from peripheral blood monocytes by culturing in the presence of GM-CSF and IL-4 together with an agent(s) known to confer tolerogenic properties. In murine systems, immature DCs are first generated by culturing bone marrow cells in the presence of GM-CSF and IL-4, and then induced to tolDCs by additional culturing in the presence of an agent(s) known to confer tolerogenic properties.15 Several pharmacological and biological agents have been used to generate tolDCs ex vivo from hematopoietic precursors or peripheral blood monocytes. The major methods used to generate tolDCs ex vivo and common features shared by the tolDCs are shown in Fig. 1.
Pharmacological agents known to induce tolDCs include vitamin D3, corticosteroid, rapamycin, cyclosporine, tacrolimus, aspirin, atorvastatin, retinoic acid, mycophenolic acid, and minocycline.1011161718192021 Of these agents, vitamin D3, dexamethasone, and rapamycin have been extensively studied in experimental animals and in humans with the aim of developing clinical approaches for the prevention of transplantation rejection and treatment of autoimmune and chronic inflammatory conditions.
The biologically active form of vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], is able to promote the generation of tolDCs.2223 DCs generated using vitamin D express lower levels of MHC class II and co-stimulatory molecules, and produce higher amount of IL-10 and lower amounts of IL-12 and IL-6, compared to untreated normal DCs.2223 Moreover, these DCs are poor activators of antigen-primed T cells, but stimulate the generation of Tregs.24 The tolDC-inducing activity of vitamin D has also been demonstrated in diabetes-prone NOD mice and normal mice.25
Corticosteroids, dexamethasone and prednisolone, have long been known to exert anti-inflammatory and immunosuppressive activities. Numerous studies have shown that corticosteroids exert their immunosuppressive activity at least in part via induction of tolDCs. DCs generated in the presence of dexamethasone express low levels of co-stimulatory molecules and MHC class II molecules, produce elevated levels of IL-10 and lower levels of IL-12, and induce the generation of Tregs.131626 Dexamethasone also induces the generation of tolerogenic macrophages.26 Moreover, DCs generated with dexamethasone retain their tolerogenicity for several days, up to a week, even after dexamethasone is removed.1626
Rapamycin has long been known to suppress T cell activation via inhibition of the serine/threonine protein kinase, mammalian target of rapamycin. Rapamycin also induces the generation of tolDCs. DCs generated with rapamycin are poor stimulators of antigen-primed T cells, resistant to maturation induced by anti-CD40 or lipopolysaccharide (LPS) stimulation, and enhance the generation of Foxp3+ Tregs.27282930 Treatment of murine heart transplantation recipients with rapamycin-generated DCs increases the survival of the transplanted organ, in correlation with increased production of Foxp3+ Tregs in the recipient mice.27
One of the drawbacks of generating tolDCs using the above listed pharmacologic agents is the cytotoxic effects of these drugs. For instance, rapamycin (10 ng/mL) is effective in generating tolDCs from bone marrow cells when used together with GM-CSF and IL-4. However, the number of CD11c+ cells obtained from rapamycin-conditioned cultures is significantly (more than 40%) lower than that from rapamycin-unconditioned cultures.27 Dexamethasone has also been shown to markedly reduce DC recovery.1626 In this regard, minocycline is unique in that it increases the generation of tolDCs from bone marrow cells.21 Minocycline also exerts growth-promoting effects on DCs conditioned with relatively toxic doses of rapamycin, vitamin D3, or IL-10.31 Furthermore, the tolerogenicity of tolDCs generated in the presence of minocycline and dexamethasone is superior or at least equal to that of tolDCs generated with either one of these agents.31
Combinations of pharmacological agents are also used to generate tolDCs with potent tolerogenic properties. For instance, potently tolerogenic and highly stable tolDCs are generated from monocytes of rheumatoid arthritis (RA) patients by the addition of dexamethasone, vitamin D3, and monophosphoryl lipid A together with GM-CSF and IL-4.32
Immunosuppressive cytokines such as IL-10 and TGF-β have been shown to induce regulatory DCs.333435 Other cytokines known to induce tolDCs include TNF-α,36 interferon (IFN)-γ,37 hepatocyte growth factor,38 and IL-21.39 TolDCs generated with IL-10 have been extensively studied in experimental animals and in humans.
DCs generated with IL-10 display reduced levels of MHC class II molecules and co-stimulatory molecules, and induce the generation of Tregs.3334354041 DCs generated with IL-10 secrete high levels of IL-10 in the absence of IL-12.34 A comparative study demonstrated that the tolerogenic properties of IL-10-generated DCs are superior to those of vitamin D3-, dexamethasone-, or rapamycin-generated DCs.40 In addition, IL-10 in combination with TGF-β induce the generation of tolDCs with potent tolerogenic properties.
The fact that IFN-γ, a prototype of the Th1-type cytokine produced mainly by natural killer (NK) and T cells, induces the generation of tolDCs is somewhat surprising. At a low dose, IFN-γ promotes the maturation of DCs with full activating potential, however, a high dose of IFN-γ induces DC acquisition of regulatory features.42 The importance of the timing and intensity of IFN-γ exposure for the function of monocyte-derived DCs (mo-DCs) was also noted in a separate study.43 A dose-dependent and bivalent effect of IFN-γ on DC function would constitue a novel mechanism for homeostatic regulation of immune responses at local sites.
Genetic engineering of DCs to express immunosuppressive molecules is a method for generating tolDCs. DCs engineered to express IL-10 using a retroviral vector exhibit significantly reduced capacity to induce allogeneic T cell proliferation and cytotoxic T lymphocyte (CTL) generation.44 Over expression of TGF-β also promotes the tolerogenic potential of the DCs.45 DCs transduced with cDNA encoding CTLA-4-Ig demonstrate markedly reduced expression of co-stimulatory molecule CD86, but not MHC class II molecules, and induce antigen-specific hyporesponsiveness.46 DCs engineered to express indoleamine 2,3-dioxygenase (IDO) or Fas ligand (FasL) also exhibit tolerogenic properties.4748
Modulation of microRNA expression in DCs is another approach for generating tolDCs. Inhibition of miRNA let-7i in DCs results in low surface expression of co-stimulatory molecules, impaired T cell stimulatory capacity, and promotion of Treg induction.49 DCs transfected with miR-23b show decreased antigen uptake, increased IL-10 production, decreased IL-12 production, and an enhanced capacity to promote Treg differentiation.50
The mechanisms by which tolDCs exert their activity are varied and incompletely understood. Moreover, phenotypic and functional differences among tolDCs arise intrinsically because of differences in the methods used to generate them. Nevertheless, there are common features shared by tolDCs, they exert an immature phenotype, and are resistant to maturation stimuli. The major mechanisms underlying the tolerance-inducing activity of tolDCs are reduction of co-stimulatory molecules, expression of various co-inhibitory molecules, production of immunosuppressive cytokines and mediators, and induction of Tregs.
The interaction of co-stimulatory molecules, such as CD80 and CD86, on DCs with CD28 on T cells triggers a T cell-activating signal. It is generally accepted that T cells become anergic and lose their ability to proliferate during subsequent stimulation when they are stimulated with signal-1, the recognition of MHC-complexed antigenic peptide via T cell receptor, in the absence of signals delivered from CD80 and CD86.2 Reduction of the expression levels of co-stimulatory molecules is one of the hallmarks of tolDCs, regardless of the methods used to generate them. TolDCs lacking co-stimulatory molecules induce T cell anergy.345
TolDCs express increased levels of various co-inhibitory molecules such as PD-L1 and ICOSL.121351 T cells become functionally inactive following their interaction with co-inhibitory molecules. A number of tolDCs also express inhibitory Ig-like transcripts (ILTs) on their surface, which interact with MHC-I molecules, especially human leukocyte antigen (HLA)-G, and deliver negative signals to T cells. ILT3 and ILT4 are upregulated by exposing immature DCs to known immunosuppressive factors such as IL-10 and vitamin D3.5253
Production of immunosuppressive cytokines, such as IL-10 and/or TGF-β, is one of the most common features of tolDCs.11 These cytokines inhibit the production of inflammatory cytokines, such as IL-12, TNF-α, and IFN-γ, and impairs the activation of T cells and NK cells.54 In addition, these cytokines induce Treg generation. IL-10, in particular, is crucial for the induction of IL-10-secreting T regulatory type 1 cells (Tr-1) cells.5556 Other immunosuppressive mediators known to be produced by tolDCs include IDO, hemoxygenase-1, and FasL. IDO has been known to suppress T and NK cells, and also induces Treg generation.575859 FasL-expressing tolDCs induce T cell apoptosis via the Fas/FasL interaction pathways.
The ability of tolDCs to direct T cell polarization toward various types of Tregs is pivotal to their tolerogenic function. TolDCs induce several subtypes of regulatory lymphocytes such as CD4+CD25+Foxp3+ Tregs, CD25+Foxp3+/− Tr-1 cells, CD8+ Tregs, and regulatory B cells.27606162 CD4+CD25+Foxp3+ Tregs have been extensively investigated in various inflammatory diseases.636465 IL-10 and TGF-β are the major cytokines produced by tolDCs and induce Treg generation. IL-10-induced tolDCs acquire the ability to secrete IL-10, which exerts powerful anti-inflammatory effects and contributes to Treg differentiation and proliferation.66 TGF-β is unique among cytokines in that it induces Foxp3 expression and promotes Treg differentiation even in the absence of DCs.67 Foxp3+ Tregs, in turn, augments the generation and tolerogenic properties of tolDCs by suppressing DC maturation.6869
The therapeutic efficacy of ex vivo-generated tolDCs has been demonstrated in animal models of autoimmune diseases such as RA,707172 diabetes,7374 and experimental allergic encephalomyelitis,75 as well as in animal models of graft rejection.7677 Based on the successes in small animal models, several clinical trials have been completed or on-going in patients with autoimmune diseases such as RA, type 1 diabetes, multiple sclerosis (MS), and Crohn's disease. Five complete clinical trials with reported outcomes are summarized in Table 1.
The first trial for RA treatment was performed with tolDCs generated from monocytes by adding the NF-κB inhibitor, BAY 11-7082.78 The tolDCs were exposed to four citrullinated peptide antigens, collagen type II1237–1249—Cit1240, fibrinogen α chain717–725—Cit720, fibrinogen β chain433–441—Cit436, and vimentin447–455—Cit450, and then administered once via intradermal injection. The results showed that a single intradermal injection of tolDCs was safe, and effective in HLA risk genotype-positive RA patients. Another clinical trial for RA treatment was performed with tolDCs generated from monocytes by adding dexamethasone, vitamin D3, and monophosphoryl lipid A.3279 Antigens in autologous synovial fluid were loaded with these tolDCs and then administered into an inflamed knee joint via intra-articular injection. The treatment was deemed safe and acceptable with promising outcomes. Two of the three patients receiving 3×106 tolDCs and one of the two patients receiving 10×106 tolDCs demonstrated improvement in vascularity on day 14, whereas no improvement was observed in the six patients receiving 1×106 tolDCs or the control intervention.
Two clinical trials were performed with tolDCs not loaded with particular antigens. The phase I study of autologous tolDCs in type 1 diabetes patients was performed with tolDCs generated from monocytes with antisense oligonucleotides targeting the primary transcripts of the CD40, CD80 and CD86 co-stimulatory molecules.80 The treatment appeared safe and well tolerated. However, there was no indication of efficacy, although an increase in potentially beneficial B220+ CD11c− B cells was observed. Another clinical trial with antigen-unloaded tolDCs was performed in patients with Crohn's disease.81 In this study, tolDCs were generated from monocytes using dexamethasone and vitamin A. The treatment appeared safe and well tolerated, and resulted in clinical improvement in 33% of the patients.
Recently, a phase 1b clinical trial with antigen-loaded tolDCs was completed in patients with MS and neuromyelitis optica.82 In this study, tolDCs were generated from monocytes with dexamethasone, and loaded with disease relevant peptides, i.e., MOG1–20, MOG35–55, MBP13–32, MBP83–99, MBP111–129, MBP146–170, PLP139–154 for MS and AQP463–76 for neuromyelitis optica. The treatment was well-tolerated, and supported the functional tolerogenic efficacy of the therapy as demonstrated by a switch towards Th2 responses, an increase in IL-10 production, and a decrease in IFN-γ production.
The primary purposes of the clinical trials currently registered with ClinicalTrials.gov are summarized in Table 2. The current on-going clinical trials with tolDCs mostly involve autoimmune diseases such as Crohn's disease, RA, MS, and type 1 diabetes mellitus. One clinical trial aiming to reduce the need for conventional immunosuppression in transplant recipients is also underway. As shown in Table 2, different approaches are being used to generate tolDCs for clinical use, raising the need to establish a more standardized ex vivo generation method(s). In fact, there are numerous questions that have to be addressed in order to achieve generalized and successful application of tolDCs in clinical settings including optimization of dose, route, and frequency of administration. Nevertheless, the list in Table 2 show that tolDC-based treatment of autoimmune diseases is now a reality, and could constitute an innovative cellular therapy in the future.
TolDCs can be generated ex vivo from peripheral blood monocytes or bone marrow cells by culturing them in the presence of GM-CSF, IL-4, and an agent(s) known to confer tolerogenic properties. The agents used extensively to generate tolDCs include vitamin D, dexamethasone, rapamycin, and IL-10, and new agents, such as minocycline, are being continuously explored. Although the mechanisms by which tolDCs exert their activity are diverse and incompletely understood, there are common features shared by tolDCs. In general, tolDCs exert an immature phenotype, and are resistant to maturation stimuli. TolDCs are characterized by reduced expression of co-stimulatory molecules, increased expression of co-inhibitory molecules, production of immunosuppressive cytokines and mediators, and/or induction of Tregs. Based on the successes in small animal models, several clinical trials have been completed or are on-going in patients with autoimmune diseases such as RA, type 1 diabetes, MS, and Crohn's disease. The results thus far are highly encouraging both in terms of safety and clinical efficacy in all the clinical studies completed to date, tolDC administration is tolerated and appears safe. More importantly, the completed clinical trials indicate significant promise for tolDC-based immunotherapy. However, numerous questions remain to be addressed prior to generalized and successful application of tolDCs in clinical settings. One of the major challenges facing tolDC-based immunotherapy is protocol optimization in order to obtain a maximum number of tolDCs with stable tolerogenic properties. In addition, the dose, route, and frequency of administration of each type of tolDC also require optimization. However, as demonstrated by numerous on-going clinical studies, tolDC-based treatment of autoimmune diseases is now a reality, and could provide innovative cellular therapy in the future.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MIST) (NRF-2017R1A2B4006828).
References
1. Haniffa M, Collin M, Ginhoux F. Ontogeny and functional specialization of dendritic cells in human and mouse. Adv Immunol. 2013; 120:1–49. PMID: 24070379.

2. Appleman LJ, Boussiotis VA. T cell anergy and costimulation. Immunol Rev. 2003; 192:161–180. PMID: 12670403.

3. Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010; 108:111–165. PMID: 21056730.

4. Raker VK, Domogalla MP, Steinbrink K. Tolerogenic dendritic cells for regulatory T cell induction in man. Front Immunol. 2015; 6:569. PMID: 26617604.

5. Lutz MB. Induction of CD4(+) regulatory and polarized effector/helper T cells by dendritic cells. Immune Netw. 2016; 16:13–25. PMID: 26937228.
6. Horton C, Shanmugarajah K, Fairchild PJ. Harnessing the properties of dendritic cells in the pursuit of immunological tolerance. Biomed J. 2017; 40:80–93. PMID: 28521905.

7. Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003; 21:685–711. PMID: 12615891.

8. Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001; 193:233–238. PMID: 11208863.

9. Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8(+) regulatory T cells in vivo in humans. Blood. 2002; 100:174–177. PMID: 12070024.

10. Svajger U, Obermajer N, Jeras M. Novel findings in drug-induced dendritic cell tolerogenicity. Int Rev Immunol. 2010; 29:574–607. PMID: 21073328.
11. Gordon JR, Ma Y, Churchman L, Gordon SA, Dawicki W. Regulatory dendritic cells for immunotherapy in immunologic diseases. Front Immunol. 2014; 5:7. PMID: 24550907.

12. Tuettenberg A, Huter E, Hubo M, Horn J, Knop J, Grimbacher B, et al. The role of ICOS in directing T cell responses: ICOS-dependent induction of T cell anergy by tolerogenic dendritic cells. J Immunol. 2009; 182:3349–3356. PMID: 19265111.

13. Unger WW, Laban S, Kleijwegt FS, van der, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009; 39:3147–3159. PMID: 19688742.
14. Domogalla MP, Rostan PV, Raker VK, Steinbrink K. Tolerance through education: how tolerogenic dendritic cells shape immunity. Front Immunol. 2017; 8:1764. PMID: 29375543.

15. Yoo S, Ha SJ. Generation of tolerogenic dendritic cells and their therapeutic applications. Immune Netw. 2016; 16:52–60. PMID: 26937232.

16. Piemonti L, Monti P, Allavena P, Sironi M, Soldini L, Leone BE, et al. Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol. 1999; 162:6473–6481. PMID: 10352262.
17. Lagaraine C, Hoarau C, Chabot V, Velge-Roussel F, Lebranchu Y. Mycophenolic acid-treated human dendritic cells have a mature migratory phenotype and inhibit allogeneic responses via direct and indirect pathways. Int Immunol. 2005; 17:351–363. PMID: 15710908.

18. Woltman AM, de Fijter JW, Kamerling SW, Paul LC, Daha MR, van Kooten C. The effect of calcineurin inhibitors and corticosteroids on the differentiation of human dendritic cells. Eur J Immunol. 2000; 30:1807–1812. PMID: 10940869.

19. Li XL, Liu Y, Cao LL, Li H, Yue LT, Wang S, et al. Atorvastatin-modified dendritic cells in vitro ameliorate experimental autoimmune myasthenia gravis by up-regulated Treg cells and shifted Th1/Th17 to Th2 cytokines. Mol Cell Neurosci. 2013; 56:85–95. PMID: 23541702.

20. Bhatt S, Qin J, Bennett C, Qian S, Fung JJ, Hamilton TA, et al. All-trans retinoic acid induces arginase-1 and inducible nitric oxide synthase-producing dendritic cells with T cell inhibitory function. J Immunol. 2014; 192:5098–5108. PMID: 24790153.
21. Kim N, Park CS, Im SA, Kim JW, Lee JH, Park YJ, et al. Minocycline promotes the generation of dendritic cells with regulatory properties. Oncotarget. 2016; 7:52818–52831. PMID: 27463004.

22. Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000; 164:4443–4451. PMID: 10779743.
23. Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A. 2001; 98:6800–6805. PMID: 11371626.
24. Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005; 106:3490–3497. PMID: 16030186.
25. Ferreira GB, Gysemans CA, Demengeot J, da Cunha JP, Vanherwegen AS, Overbergh L, et al. 1,25-Dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. J Immunol. 2014; 192:4210–4220. PMID: 24663679.

26. Xia CQ, Peng R, Beato F, Clare-Salzler MJ. Dexamethasone induces IL-10-producing monocyte-derived dendritic cells with durable immaturity. Scand J Immunol. 2005; 62:45–54. PMID: 16091124.

27. Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007; 178:7018–7031. PMID: 17513751.
28. Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, et al. Rapamycin inhibits IL-4--induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003; 101:4457–4463. PMID: 12531798.

29. Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005; 105:4743–4748. PMID: 15746082.
30. Taner T, Hackstein H, Wang Z, Morelli AE, Thomson AW. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce ag-specific T cell regulation and prolong graft survival. Am J Transplant. 2005; 5:228–236. PMID: 15643982.

31. Lee JH, Park CS, Jang S, Kim JW, Kim SH, Song S, et al. Tolerogenic dendritic cells are efficiently generated using minocycline and dexamethasone. Sci Rep. 2017; 7:15087. PMID: 29118423.

32. Harry RA, Anderson AE, Isaacs JD, Hilkens CM. Generation and characterisation of therapeutic tolerogenic dendritic cells for rheumatoid arthritis. Ann Rheum Dis. 2010; 69:2042–2050. PMID: 20551157.

33. Torres-Aguilar H, Aguilar-Ruiz SR, González-Pérez G, Munguía R, Bajaña S, Meraz-Ríos MA, et al. Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4+ T cells. J Immunol. 2010; 184:1765–1775. PMID: 20083662.
34. Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010; 116:935–944. PMID: 20448110.

35. Nayyar A, Dawicki W, Huang H, Lu M, Zhang X, Gordon JR. Induction of prolonged asthma tolerance by IL-10-differentiated dendritic cells: differential impact on airway hyperresponsiveness and the Th2 immunoinflammatory response. J Immunol. 2012; 189:72–79. PMID: 22634620.

36. Menges M, Rössner S, Voigtländer C, Schindler H, Kukutsch NA, Bogdan C, et al. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002; 195:15–21. PMID: 11781361.
37. Eljaafari A, Li YP, Miossec P. IFN-gamma, as secreted during an alloresponse, induces differentiation of monocytes into tolerogenic dendritic cells, resulting in FoxP3+ regulatory T cell promotion. J Immunol. 2009; 183:2932–2945. PMID: 19696431.
38. Rutella S, Bonanno G, Procoli A, Mariotti A, de Ritis DG, Curti A, et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood. 2006; 108:218–227. PMID: 16527888.
39. Brandt K, Bulfone-Paus S, Foster DC, Rückert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. 2003; 102:4090–4098. PMID: 12893770.

40. Boks MA, Kager-Groenland JR, Haasjes MS, Zwaginga JJ, van Ham SM, ten Brinke A. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction--a comparative study of human clinical-applicable DC. Clin Immunol. 2012; 142:332–342. PMID: 22225835.
41. Kryczanowsky F, Raker V, Graulich E, Domogalla MP, Steinbrink K. IL-10-modulated human dendritic cells for clinical use: identification of a stable and migratory subset with improved tolerogenic activity. J Immunol. 2016; 197:3607–3617. PMID: 27683749.

42. Svajger U, Obermajer N, Jeras M. IFN-γ-rich environment programs dendritic cells toward silencing of cytotoxic immune responses. J Leukoc Biol. 2014; 95:33–46. PMID: 23924658.

43. Kerkar SP, Chinnasamy D, Hadi N, Melenhorst J, Muranski P, Spyridonidis A, et al. Timing and intensity of exposure to interferon-γ critically determines the function of monocyte-derived dendritic cells. Immunology. 2014; 143:96–108. PMID: 24678989.
44. Takayama T, Nishioka Y, Lu L, Lotze MT, Tahara H, Thomson AW. Retroviral delivery of viral interleukin-10 into myeloid dendritic cells markedly inhibits their allostimulatory activity and promotes the induction of T-cell hyporesponsiveness. Transplantation. 1998; 66:1567–1574. PMID: 9884241.
45. Lee WC, Zhong C, Qian S, Wan Y, Gauldie J, Mi Z, et al. Phenotype, function, and in vivo migration and survival of allogeneic dendritic cell progenitors genetically engineered to express TGF-beta. Transplantation. 1998; 66:1810–1817. PMID: 9884280.
46. Lu L, Gambotto A, Lee WC, Qian S, Bonham CA, Robbins PD, et al. Adenoviral delivery of CTLA4Ig into myeloid dendritic cells promotes their in vitro tolerogenicity and survival in allogeneic recipients. Gene Ther. 1999; 6:554–563. PMID: 10476215.

47. Bianco NR, Kim SH, Ruffner MA, Robbins PD. Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase-positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheum. 2009; 60:380–389. PMID: 19180475.

48. Kim SH, Kim S, Oligino TJ, Robbins PD. Effective treatment of established mouse collagen-induced arthritis by systemic administration of dendritic cells genetically modified to express FasL. Mol Ther. 2002; 6:584–590. PMID: 12409256.

49. Zhang M, Liu F, Jia H, Zhang Q, Yin L, Liu W, et al. Inhibition of microRNA let-7i depresses maturation and functional state of dendritic cells in response to lipopolysaccharide stimulation via targeting suppressor of cytokine signaling 1. J Immunol. 2011; 187:1674–1683. PMID: 21742974.

50. Zheng J, Jiang HY, Li J, Tang HC, Zhang XM, Wang XR, et al. MicroRNA-23b promotes tolerogenic properties of dendritic cells in vitro through inhibiting Notch1/NF-κB signalling pathways. Allergy. 2012; 67:362–370. PMID: 22229716.

51. Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004; 101:10691–10696. PMID: 15249675.

52. Vlad G, Chang CC, Colovai AI, Vasilescu ER, Cortesini R, Suciu-Foca N. Membrane and soluble ILT3 are critical to the generation of T suppressor cells and induction of immunological tolerance. Int Rev Immunol. 2010; 29:119–132. PMID: 20132030.

53. Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002; 3:237–243. PMID: 11875462.

54. Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009; 9:447–453. PMID: 19481975.
55. Battaglia M, Stabilini A, Draghici E, Gregori S, Mocchetti C, Bonifacio E, et al. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes. 2006; 55:40–49. PMID: 16380475.

56. Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005; 105:1162–1169. PMID: 15479730.
57. Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002; 196:459–468. PMID: 12186838.

58. Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002; 297:1867–1870. PMID: 12228717.

59. Mellor AL, Baban B, Chandler P, Marshall B, Jhaver K, Hansen A, et al. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol. 2003; 171:1652–1655. PMID: 12902462.

60. Huang H, Dawicki W, Zhang X, Town J, Gordon JR. Tolerogenic dendritic cells induce CD4+CD25hiFoxp3+ regulatory T cell differentiation from CD4+CD25−/loFoxp3− effector T cells. J Immunol. 2010; 185:5003–5010. PMID: 20870943.
61. Qian L, Qian C, Chen Y, Bai Y, Bao Y, Lu L, et al. Regulatory dendritic cells program B cells to differentiate into CD19hiFcγIIbhi regulatory B cells through IFN-β and CD40L. Blood. 2012; 120:581–591. PMID: 22692512.
62. Hsu SM, Mathew R, Taylor AW, Stein-Streilein J. Ex-vivo tolerogenic F4/80+ antigen-presenting cells (APC) induce efferent CD8+regulatory T cell-dependent suppression of experimental autoimmune uveitis. Clin Exp Immunol. 2014; 176:37–48. PMID: 24266626.
63. Barbi J, Pardoll D, Pan F. Treg functional stability and its responsiveness to the microenvironment. Immunol Rev. 2014; 259:115–139. PMID: 24712463.

64. Hori S. Lineage stability and phenotypic plasticity of Foxp3+ regulatory T cells. Immunol Rev. 2014; 259:159–172. PMID: 24712465.
65. Jung MK, Kwak JE, Shin EC. IL-17A-producing Foxp3+ regulatory T cells and human diseases. Immune Netw. 2017; 17:276–286. PMID: 29093649.
66. Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001; 2:725–731. PMID: 11477409.

67. Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003; 198:1875–1886. PMID: 14676299.
68. Mahnke K, Johnson TS, Ring S, Enk AH. Tolerogenic dendritic cells and regulatory T cells: a two-way relationship. J Dermatol Sci. 2007; 46:159–167. PMID: 17428639.

69. Kornete M, Piccirillo CA. Functional crosstalk between dendritic cells and Foxp3(+) regulatory T cells in the maintenance of immune tolerance. Front Immunol. 2012; 3:165. PMID: 22737152.

70. Popov I, Li M, Zheng X, San H, Zhang X, Ichim TE, et al. Preventing autoimmune arthritis using antigen-specific immature dendritic cells: a novel tolerogenic vaccine. Arthritis Res Ther. 2006; 8:R141. PMID: 16911769.
71. van Duivenvoorde LM, Han WG, Bakker AM, Louis-Plence P, Charbonnier LM, Apparailly F, et al. Immunomodulatory dendritic cells inhibit Th1 responses and arthritis via different mechanisms. J Immunol. 2007; 179:1506–1515. PMID: 17641016.

72. Ahmed MS, Bae YS. Dendritic cell-based immunotherapy for rheumatoid arthritis: from bench to bedside. Immune Netw. 2016; 16:44–51. PMID: 26937231.

73. Phillips BE, Giannoukakis N, Trucco M. Dendritic cell mediated therapy for immunoregulation of type 1 diabetes mellitus. Pediatr Endocrinol Rev. 2008; 5:873–879. PMID: 18552749.
74. Tai N, Yasuda H, Xiang Y, Zhang L, Rodriguez-Pinto D, Yokono K, et al. IL-10-conditioned dendritic cells prevent autoimmune diabetes in NOD and humanized HLA-DQ8/RIP-B7.1 mice. Clin Immunol. 2011; 139:336–349. PMID: 21458378.

75. Chorny A, Gonzalez-Rey E, Fernandez-Martin A, Pozo D, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc Natl Acad Sci U S A. 2005; 102:13562–13567. PMID: 16150720.

76. Horibe EK, Sacks J, Unadkat J, Raimondi G, Wang Z, Ikeguchi R, et al. Rapamycin-conditioned, alloantigen-pulsed dendritic cells promote indefinite survival of vascularized skin allografts in association with T regulatory cell expansion. Transpl Immunol. 2008; 18:307–318. PMID: 18158116.

77. Raimondi G, Sumpter TL, Matta BM, Pillai M, Corbitt N, Vodovotz Y, et al. Mammalian target of rapamycin inhibition and alloantigen-specific regulatory T cells synergize to promote long-term graft survival in immunocompetent recipients. J Immunol. 2010; 184:624–636. PMID: 20007530.

78. Benham H, Nel HJ, Law SC, Mehdi AM, Street S, Ramnoruth N, et al. Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci Transl Med. 2015; 7:290ra87.

79. Bell GM, Anderson AE, Diboll J, Reece R, Eltherington O, Harry RA, et al. Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann Rheum Dis. 2017; 76:227–234. PMID: 27117700.

80. Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011; 34:2026–2032. PMID: 21680720.

81. Jauregui-Amezaga A, Cabezón R, Ramírez-Morros A, España C, Rimola J, Bru C, et al. Intraperitoneal administration of autologous tolerogenic dendritic cells for refractory Crohn's disease: a phase I study. J Crohns Colitis. 2015; 9:1071–1078. PMID: 26303633.

82. Nafarrate IZ, Florez G, Vila G, Cabezón R, España C, Benitez D, et al. Phase 1b clinical trial with antigen-specific tolerogenic dendritic in Multiple Sclerosis and Neuromyelitis optica: safety and immunological effects (P2.330). Neurology. 2017; 88(16 Supplement):P2.330.
Fig. 1
Generation, characteristics, and mechanisms of action of tolDCs. Human tolDCs are mostly produced from peripheral blood monocytes by culturing with GM-CSF, IL-4, and an agent(s) known to confer tolerogenic properties. In murine systems, immature DCs are first generated by culturing bone marrow cells with GM-CSF and IL-4, and then induced to tolDCs by additional culturing with an agent(s) known to confer tolerogenic properties. TolDCs induce several subtypes of regulatory lymphocytes such as CD4+CD25+Foxp3+ Tregs, and CD25+Foxp3+/− Tr-1 cells from precursor T cells (pTh). DC, dendritic cell; tolDCs, tolerogenic DCs; GM-CSF, granulocyte macrophage-colony stimulating factor; IL, interleukin; MHC, major histocompatibility complex; PD-L1, programmed death-ligand 1; ICOSL, inducible costimulator ligand; TNF, tumor necrosis factor; IFN, interferon; TLR, toll-like receptor; IDO, indoleamine 2,3-dioxygenase; FasL, Fas ligand; TGF, transforming growth factor.
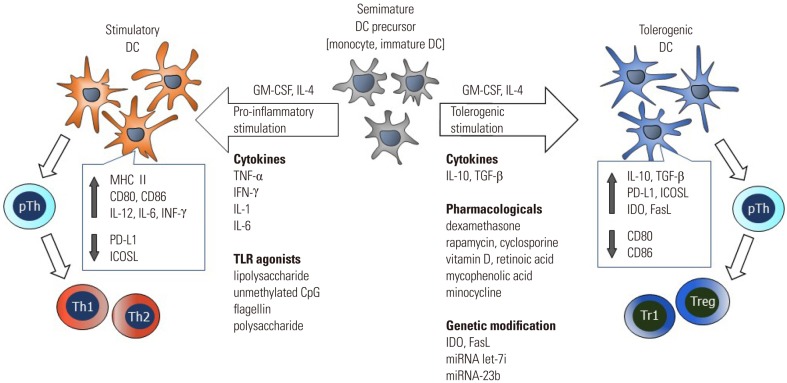
Table 1
Completed Clinical Trials with Reported Outcomes
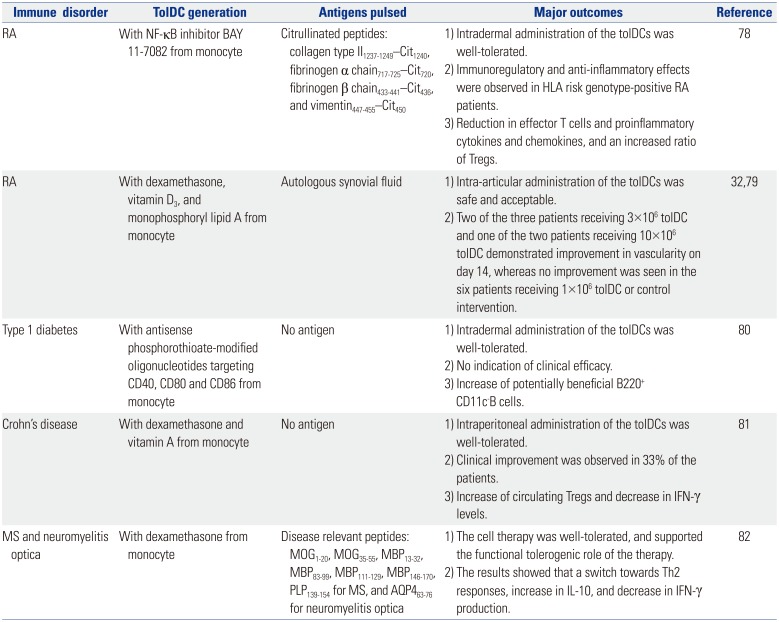
| Immune disorder | TolDC generation | Antigens pulsed | Major outcomes | Reference |
|---|---|---|---|---|
| RA | With NF-κB inhibitor BAY 11-7082 from monocyte | Citrullinated peptides: collagen type II1237-1249–Cit1240, fibrinogen α chain717-725–Cit720, fibrinogen β chain433-441–Cit436, and vimentin447-455–Cit450 | 1) Intradermal administration of the tolDCs was well-tolerated. | 78 |
| 2) Immunoregulatory and anti-inflammatory effects were observed in HLA risk genotype-positive RA patients. | ||||
| 3) Reduction in effector T cells and proinflammatory cytokines and chemokines, and an increased ratio of Tregs. | ||||
| RA | With dexamethasone, vitamin D3, and monophosphoryl lipid A from monocyte | Autologous synovial fluid | 1) Intra-articular administration of the tolDCs was safe and acceptable. | 3279 |
| 2) Two of the three patients receiving 3×106 tolDC and one of the two patients receiving 10×106 tolDC demonstrated improvement in vascularity on day 14, whereas no improvement was seen in the six patients receiving 1×106 tolDC or control intervention. | ||||
| Type 1 diabetes | With antisense phosphorothioate-modified oligonucleotides targeting CD40, CD80 and CD86 from monocyte | No antigen | 1) Intradermal administration of the tolDCs was well-tolerated. | 80 |
| 2) No indication of clinical efficacy. | ||||
| 3) Increase of potentially beneficial B220+ CD11c-Bcells. | ||||
| Crohn's disease | With dexamethasone and vitamin A from monocyte | No antigen | 1) Intraperitoneal administration of the tolDCs was well-tolerated. | 81 |
| 2) Clinical improvement was observed in 33% of the patients. | ||||
| 3) Increase of circulating Tregs and decrease in IFN-γ levels. | ||||
| MS and neuromyelitis optica | With dexamethasone from monocyte | Disease relevant peptides: MOG1-20, MOG35-55, MBP13-32, MBP83-99, MBP111-129, MBP146-170, PLP139-154 for MS, and AQP463-76 for neuromyelitis optica | 1) The cell therapy was well-tolerated, and supported the functional tolerogenic role of the therapy. | 82 |
| 2) The results showed that a switch towards Th2 responses, increase in IL-10, and decrease in IFN-γ production. |
Table 2
Clinical Trials Currently Registered at ClinicalTrials.gov
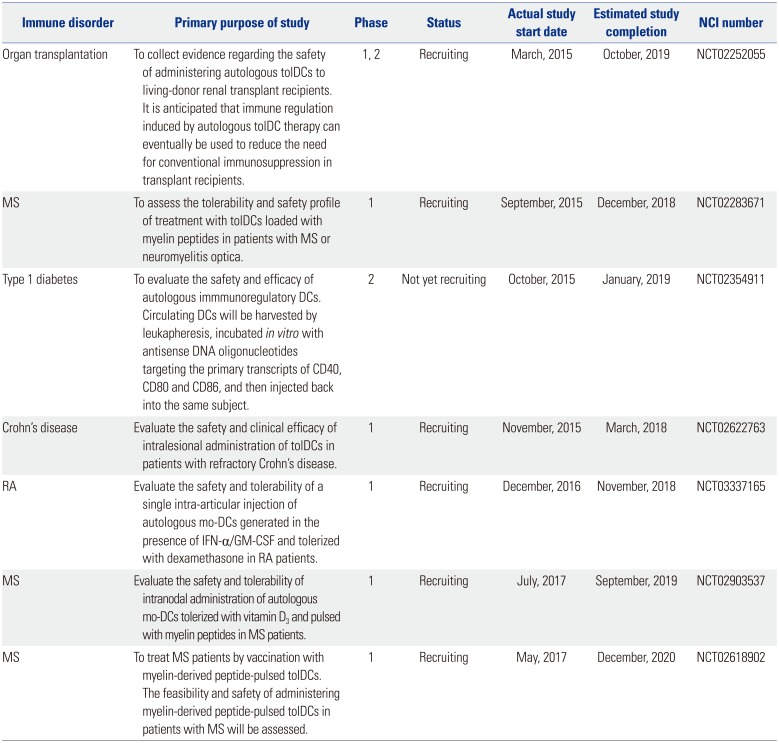
| Immune disorder | Primary purpose of study | Phase | Status | Actual study start date | Estimated study completion | NCI number |
|---|---|---|---|---|---|---|
| Organ transplantation | To collect evidence regarding the safety of administering autologous tolDCs to living-donor renal transplant recipients. It is anticipated that immune regulation induced by autologous tolDC therapy can eventually be used to reduce the need for conventional immunosuppression in transplant recipients. | 1, 2 | Recruiting | March, 2015 | October, 2019 | NCT02252055 |
| MS | To assess the tolerability and safety profile of treatment with tolDCs loaded with myelin peptides in patients with MS or neuromyelitis optica. | 1 | Recruiting | September, 2015 | December, 2018 | NCT02283671 |
| Type 1 diabetes | To evaluate the safety and efficacy of autologous immmunoregulatory DCs. Circulating DCs will be harvested by leukapheresis, incubated in vitro with antisense DNA oligonucleotides targeting the primary transcripts of CD40, CD80 and CD86, and then injected back into the same subject. | 2 | Not yet recruiting | October, 2015 | January, 2019 | NCT02354911 |
| Crohn's disease | Evaluate the safety and clinical efficacy of intralesional administration of tolDCs in patients with refractory Crohn's disease. | 1 | Recruiting | November, 2015 | March, 2018 | NCT02622763 |
| RA | Evaluate the safety and tolerability of a single intra-articular injection of autologous mo-DCs generated in the presence of IFN-α/GM-CSF and tolerized with dexamethasone in RA patients. | 1 | Recruiting | December, 2016 | November, 2018 | NCT03337165 |
| MS | Evaluate the safety and tolerability of intranodal administration of autologous mo-DCs tolerized with vitamin D3 and pulsed with myelin peptides in MS patients. | 1 | Recruiting | July, 2017 | September, 2019 | NCT02903537 |
| MS | To treat MS patients by vaccination with myelin-derived peptide-pulsed tolDCs. The feasibility and safety of administering myelin-derived peptide-pulsed tolDCs in patients with MS will be assessed. | 1 | Recruiting | May, 2017 | December, 2020 | NCT02618902 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download