Abstract
Purpose
The purpose of this study was to evaluate the effects of lifestyle behaviors and health habits on the risk for acquiring pandemic influenza (H1N1) virus infection.
Materials and Methods
We conducted a case-control study in a secondary care hospital in South Korea between November 2009 and August 2010. We enrolled patients with H1N1 infection, as confirmed by a positive result of the real-time reverse transcriptase polymerase chain reaction assay; for each patient, we enrolled 4 age- and gender-matched controls with no history of H1N1 infection or severe acute respiratory illness during the H1N1 pandemic in South Korea (1:4 match).
Results
During the study period, 33 cases and 132 age- and gender-matched controls were enrolled. The case group had a higher percentage of current smokers (p<0.01), fewer subjects reporting regular physical activity (p=0.03), or regular vitamin supplementation (p<0.01), and more subjects reporting a higher annual incidence of the common cold (p=0.048) as compared to the control group. In the multivariable analysis, 2 factors were independently associated with the acquisition of H1N1 infection: current smoking [adjusted odds ratio (OR)=5.53; 95% confidence interval (CI), 1.60-19.16; p<0.01] and a higher annual incidence of the common cold (adjusted OR=1.24; 95% CI, 1.002-1.53; p=0.048).
A novel strain of influenza A (H1N1), identified in Mexico and the United States (USA) in 2009, spread rapidly worldwide, and many countries experienced the first wave of infection between spring and the end of 2009,1 and the cumulative number of people infected also increased rapidly. The Centers for Disease Control and Prevention estimated 60 million cases of pandemic H1N1 in the USA since the spring of 2009.2
A series of teleconferences organized by the World Health Organization concluded that the risk factors for severe disease following H1N1 virus infection were similar to those for seasonal influenza infection, e.g., chronic medical conditions, certain cognitive conditions, and immunodeficiency.3 In addition, several new risk factors such as pregnancy,4 obesity, and tuberculosis5,6 were also noted. However, data on the risk factors for increased susceptibility to H1N1 infection in a healthy population are scarce.
Lifestyle behaviors such as cigarette smoking, physical activity, and stress have an impact on respiratory infections; cigarette smoking alone is known to be a major risk factor. The basis for increased susceptibility to infections in smokers is multifactorial, and one of the mechanisms is alteration of structural and immunological defenses.7 An association between physical activity and upper respiratory infection has also been documented. Nieman suggested that moderate levels of physical activity reduce the risk of upper respiratory tract infection.8 In addition, chronic stress is known to be associated with suppression of both cellular and humoral immune function,9 and self-reported perceived stress is associated with an increased risk of upper respiratory tract infection.10 To our knowledge, however, there has been thus far no research on the relationship between lifestyle behaviors and H1N1 infection.
The purpose of this study was to determine whether lifestyle behaviors (smoking, alcohol consumption, physical activity, and sleep duration) and health habits (use of vitamin supplements) are risk factors for H1N1 viral infection.
A case-control study was conducted at the Seoul Metropolitan Government Seoul National University Boramae Medical Center, a secondary care hospital in South Korea. Between November 2009 and March 2010, we recruited patients who visited the hospital because of suspicion of influenza. The inclusion criteria were age ≥18 years and a pandemic 2009 influenza (H1N1) virus infection confirmed by a positive result of real-time reverse transcriptase polymerase chain reaction using nasopharyngeal or oropharyngeal swab samples. We recruited 4 age- and gender-matched controls for each confirmed case of H1N1 influenza from among high school and college students, healthcare personnel, and adults who visited the same hospital for a health screening between June and August 2010. Control subjects reported that they had no history of H1N1 infection or severe acute respiratory illness from September 2009 to March 2010 (the period of the H1N1 pandemic in South Korea). All participants signed a written informed consent form and agreed to participate in the study.
All participants filled out a questionnaire, which collected demographic information such that on age, gender, height, body weight, body mass index (BMI), and co-morbidities. Participants were also asked to report whether they had been vaccinated for the H1N1 virus and to provide an estimate of their historical average frequency of common colds per year. The following information on lifestyle behaviors was collected: smoking status, alcohol intake (frequency and amount per week), physical activity (type, duration, and frequency per week), stress (numeric rating scale), and sleep duration (wake-up time and bedtime). Health habits recorded included the use of vitamin supplements (type and frequency per week) and frequency of meals per day (for nutritional status). Differences between cases and controls for the variables of lifestyle behaviors and health habits were evaluated using the Student's t-test or the Mann-Whitney U test for continuous variables and the χ2 test or Fisher's exact test for categorical variables in univariate analyses. Multivariate logistic regression analysis was also performed. A p value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and STATA 12.1 (StataCorp, College Station, TX, USA).
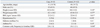
During the period of enrollment, 33 patients with diagnosed H1N1 infection and 132 age- and gender-matched controls were recruited. The median age was 41 years (range, 18-72 years) in the case group and 40 years (range, 19-72 years) in the control group; 45.5% were men in the entire population. Table 1 presents the baseline characteristics of the case and control groups. There were no differences in BMI or comorbid diseases (diabetes mellitus, hypertension, or asthma) between the 2 groups, and there were no pregnant women in the study population.
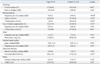
The case group had a higher annual frequency of common cold (p=0.048) (Table 1) and higher percentage of current smokers (p<0.01) (Table 2), but a lower percentage of participants performing regular physical activity (≥3 times per week) than the control group (p=0.03). The types of physical activity included weight training (23.6%), walking (20%), golf (16.4%), running (10.9%), climbing (10.9%), and swimming (9.1%). The case group also had a lower percentage of participants reporting regular vitamin supplementation (≥3 times per week) than the controls (p<0.01) (Table 2). There were no differences in the frequency of meals per day, alcohol intake, stress intensity, or sleep duration between the 2 groups (Table 2).
In the multivariate analysis, 2 variables were independently associated with the acquisition of H1N1 infection: current smoking status [adjusted odds ratio (OR)=5.53; 95% confidence interval (CI), 1.60-19.2; p<0.01] and a higher annual frequency of the common cold (adjusted OR=1.24; 95% CI, 1.00-1.53; p=0.048) (Table 3). We also conducted a sensitivity analysis for the participants without a history of vaccination for H1N1 influenza, and the results obtained were similar to those obtained for all the participants, except that the variable of vitamin supplementation ≥3 times per week was associated with a lower risk of acquiring H1N1 infection (Table 4).
The current study focused on determining whether lifestyle behaviors (smoking, alcohol consumption, physical activity, and sleep duration) and health habits (use of vitamin supplements) were risk factors for H1N1 viral infection. In this case-control study, current cigarette smoking and a higher annual frequency of common cold were associated with the acquisition of H1N1 influenza. There have been several studies evaluating the effect of lifestyle behaviors on the risk of respiratory disease, such as chronic bronchitis, emphysema, and upper respiratory infection. However, to our best knowledge, no previous studies have evaluated the effect of lifestyle behaviors on the risk for H1N1 infection.
Current smoking was a major independent risk factor for acquiring H1N1 infection in our study, consistent with those of previous studies evaluating the relationship between smoking and influenza.11 Kark and Lebiush12 showed that female smokers in the Israeli Army had a 60% risk for influenza as compared to a 41.6% risk among female nonsmokers (OR=1.44; 95% CI, 1.03-2.01), and the same authors in another study reported that 68.5% of smokers as compared to 47.2% of nonsmokers contracted H1N1 infection (p<0.01).13 The study showed that smoking was a major determinant of morbidity in an epidemic H1N1 infection, and that outbreaks of H1N1 in heavy smokers might contribute to substantial incapacitation.13 Mechanisms increasing the risk of infections in smokers include structural changes in the respiratory tract and a decrease in the immune response.7 Structural changes include peribronchiolar inflammation and fibrosis, increased mucosal permeability, impairment of mucociliary clearance, changes in pathogen adherence, and disruption of the respiratory epithelium.14 These changes may alter both susceptibility to infection and the course of an infection and compromise the ability to mount appropriate immune and inflammatory responses.15 Meliska, et al.16 and Hersey, et al.17 reported that natural killer cell cytotoxic activity increased substantially in smokers who quit smoking compared to current smokers. This could explain why current smokers have a higher risk than never- or ex-smokers.
Previous studies have reported the beneficial effects of physical activity on H1N1 infection or other upper respiratory tract infection (URTI), which was attributed to the immunological effects of exercise. These studies suggested that exercise was associated with a lesser decline in circulating T-cell function with viral clearance, specific antibody production, and neuroendocrine changes in the immune system.18-22 Interestingly, a J-curve relationship has been shown for the effects of exercise on risk for infection;8 therefore, as the amount of exercise increases, the risk first decreases but then increases after some level.8 In our study, physical activity failed to show a significant effect on H1N1 acquisition in multivariate analysis, although the controls reported more frequent physical activity than the cases in univariate analysis.
Micronutrients such as vitamins affect the susceptibility to infection, and there has been considerable interest in the association of vitamin supplementation with URTIs. Vitamin D was found to modulate the macrophage response by reducing the amount of inflammatory cytokines and chemokines released.23 A study using an influenza virus-infected vitamin C-deficient mouse model demonstrated enhanced lung pathology.24 One randomized controlled trial testing the effects of vitamin supplementation showed a lower rate of development of self-reported cold and influenza symptoms.25 Although our study did not show a significant effect of vitamin supplementation on H1N1 acquisition, vitamin supplementation ≥3 times per week prevented H1N1 acquisition (OR=0.251; 95% CI, 0.07-0.96; p=0.043) (Table 4) in the sensitivity analysis for the participants without a history of vaccination for H1N1 influenza. However, it should be acknowledged that the statistical significance of the effect of vitamin supplementation on the risk for acquiring influenza might have been due to multiple testing.
Previous epidemiologic studies reported that a mean annual incidence of common colds in adults varied from 0.9 to 3.3 (illness per person-year), depending on age and gender.26,27 The frequency of common colds reported by participants of our study was consistent with the findings of previous epidemiological studies. In our study, a higher frequency of contracting the common cold was related to H1N1 infection acquisition. Hosts susceptible to common colds caused by several respiratory viruses are likely to be susceptible to H1N1 influenza virus. In addition, there is a possibility that people with frequent common colds visit the clinic more often and are more likely to be diagnosed with H1N1 infection. Therefore, we used the frequency of common colds as a confounder in the adjusted multivariable analysis.
Besides smoking, vitamin supplementation, and physical activity, several other factors have been associated with the risk of H1N1 infection. A cross-sectional survey evaluating the characteristics of H1N1 outbreaks among healthcare personnel28 showed that the female gender, an age <40 years, chronic underlying diseases, and having infected household members were associated with H1N1 infection. Obesity was also identified as a risk factor for H1N1 infection. A recent study from California reported that half of Californians aged ≥20 years, and hospitalized with H1N1 infection were obese,29 and extreme obesity (BMI >40 kg/m2) was found to be independently associated with death due to the 2009 H1N1 pandemic after adjusting for co-morbidities and other risk factors. However, this study did not show a relationship between BMI and the risk for H1N1 infection.
The present study has several limitations. First, the number of cases enrolled was small. This one-center, case-control study was initiated at a late phase of the pandemic in Korea, after receiving approval from the ethical review board. However, we attempted to compensate for this weakness by increasing the number of controls. Second, the period of enrollment was different between the cases and the controls. Whether a subject acquired H1N1 infection was better determined after the pandemic ended; therefore, control subjects were enrolled after the enrollment of the cases. This might have induced recall bias. In addition, it could have led to bias in the comparison of physical activity between the cases and controls because physical activity can vary during the year. Nevertheless, the percentages reporting outdoor exercise were similar between the cases and controls (62.5% vs. 54.5%, respectively; p>0.99). Third, there could have been healthy volunteer bias, as many control subjects were healthcare personnel or people who visited hospitals for health screening.30,31 Finally, it is possible that some of the control subjects experienced mild H1N1 infection without influenza-like symptoms, because they were enrolled by self-reporting no history of H1N1 infection; this was not confirmed by serologic testing for H1N1 infection.
In conclusion, our study showed that current smoking and a frequent history of common colds were risk factors for acquiring H1N1 infection.
Figures and Tables
References
1. World Health Organization. Evolution of a pandemic: A(H1N1) 2009, April 2009-March 2010. accessed on 2012 October 17. Available at: http://whqlibdoc.who.int/publications/2010/9789241599924_eng.pdf.
2. Centers for Disease Control Prevention. Updated CDC Estimates of 2009 H1N1 Influenza Cases, Hospitalizations and Deaths in the United States, April 2009-April 10, 2010. accessed on 2012 June 3. Available at: http://www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm.
3. World Health Organization. Clinical features of severe cases of pandemic influenza. Pandemic (H1N1) 2009 briefing note 13. accessed on 2012 October 20. Available at: http://www.who.int/csr/disease/swineflu/notes/h1n1_clinical_features_20091016/en/index.html.
4. Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009; 374:451–458.

5. Fezeu L, Julia C, Henegar A, Bitu J, Hu FB, Grobbee DE, et al. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obes Rev. 2011; 12:653–659.

6. Archer B, Cohen C, Naidoo D, Thomas J, Makunga C, Blumberg L, et al. Interim report on pandemic H1N1 influenza virus infections in South Africa, April to October 2009: epidemiology and factors associated with fatal cases. Euro Surveill. 2009; 14.

8. Nieman DC. Exercise, upper respiratory tract infection, and the immune system. Med Sci Sports Exerc. 1994; 26:128–139.

9. Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004; 130:601–630.

10. Cohen S. Keynote Presentation at the Eight International Congress of Behavioral Medicine: the Pittsburgh common cold studies: psychosocial predictors of susceptibility to respiratory infectious illness. Int J Behav Med. 2005; 12:123–131.

11. Finklea JF, Sandifer SH, Smith DD. Cigarette smoking and epidemic influenza. Am J Epidemiol. 1969; 90:390–399.
12. Kark JD, Lebiush M. Smoking and epidemic influenza-like illness in female military recruits: a brief survey. Am J Public Health. 1981; 71:530–532.

13. Kark JD, Lebiush M, Rannon L. Cigarette smoking as a risk factor for epidemic a(h1n1) influenza in young men. N Engl J Med. 1982; 307:1042–1046.

14. Dye JA, Adler KB. Effects of cigarette smoke on epithelial cells of the respiratory tract. Thorax. 1994; 49:825–834.

15. Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009; 9:377–384.

16. Meliska CJ, Stunkard ME, Gilbert DG, Jensen RA, Martinko JM. Immune function in cigarette smokers who quit smoking for 31 days. J Allergy Clin Immunol. 1995; 95:901–910.

17. Hersey P, Prendergast D, Edwards A. Effects of cigarette smoking on the immune system. Follow-up studies in normal subjects after cessation of smoking. Med J Aust. 1983; 2:425–429.

18. Liu YG, Wang SY. The enhancing effect of exercise on the production of antibody to Salmonella typhi in mice. Immunol Lett. 1987; 14:117–120.

19. Kaufman JC, Harris TJ, Higgins J, Maisel AS. Exercise-induced enhancement of immune function in the rat. Circulation. 1994; 90:525–532.

20. Shinkai S, Kohno H, Kimura K, Komura T, Asai H, Inai R, et al. Physical activity and immune senescence in men. Med Sci Sports Exerc. 1995; 27:1516–1526.

21. Schuler PB, Leblanc PA, Marzilli TS. Effect of physical activity on the production of specific antibody in response to the 1998-99 influenza virus vaccine in older adults. J Sports Med Phys Fitness. 2003; 43:404.
22. Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000; 80:1055–1081.

23. Helming L, Böse J, Ehrchen J, Schiebe S, Frahm T, Geffers R, et al. 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood. 2005; 106:4351–4358.

24. Li W, Maeda N, Beck MA. Vitamin C deficiency increases the lung pathology of influenza virus-infected gulo-/- mice. J Nutr. 2006; 136:2611–2616.

25. Aloia JF, Li-Ng M. Re: epidemic influenza and vitamin D. Epidemiol Infect. 2007; 135:1095–1096.
26. Fox JP, Hall CE, Cooney MK, Luce RE, Kronmal RA. The Seattle virus watch. II. Objectives, study population and its observation, data processing and summary of illnesses. Am J Epidemiol. 1972; 96:270–285.

27. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974; 227:164–169.

28. Choi SH, Chung JW, Jeon MH, Lee MS. Risk factors for pandemic H1N1 2009 infection in healthcare personnel of four general hospitals. J Infect. 2011; 63:267–273.

29. Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis. 2011; 52:301–312.

30. Criqui MH, Barrett-Connor E, Austin M. Differences between respondents and non-respondents in a population-based cardiovascular disease study. Am J Epidemiol. 1978; 108:367–372.

31. Heilbrun LK, Nomura A, Stemmermann GN. The effects of nonresponse in a prospective study of cancer. Am J Epidemiol. 1982; 116:353–363.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download