INTRODUCTION
Acute kidney injury (AKI) is a common condition in critically ill patients and known to be associated with increased mortality.
1,
2 Sepsis is the systemic inflammatory response to infection, and one of the most common contributing factors in AKI of critical illness.
3 Previous studies of septic AKI noted that about 60% patients with septic shock developed AKI and found a higher mortality and longer duration of hospital stay in patients with AKI compared with patients without AKI.
4,
5
Distinctive clinical features of AKI of septic etiology have been elucidated in recent studies,
3-
6 which implies that a unique pathogenesis contributes to the development of septic AKI.
7-
10 Although recent advances in understanding of pathophysiology of septic AKI have been achieved, because of its complexity and multifactorial character, effective management for septic AKI is limited to fluid resuscitation and antibiotics.
According to the risk, injury, failure, loss of function, and end-stage renal disease (RIFLE) criteria,
11 AKI is defined by serum creatinine levels and urine output. Although small changes in serum creatinine or acute reduction in urine output can be used in the diagnosis of AKI, these changes are often evident after the chance of effective management for renal protection has already passed. A previous prospective multicenter study reported the delayed onset of AKI of septic origin compared with AKI of non-septic origin.
12 The recognition of high-risk patients for development of septic AKI is important because early interventions may improve the clinical course.
Considering its high prevalence and clinical significance, the clinical aspects of AKI in sepsis and septic shock patients should be further elucidated. Therefore, the present study aimed to investigate the clinical characteristics of AKI in patients with sepsis and septic shock and clinical risk factors of AKI in patients with septic condition. In addition, whether AKI increased the mortality and, if so, how the severity of AKI affected the clinical outcomes was analyzed.
DISCUSSION
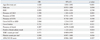
Among 992 patients with sepsis and septic shock, 573 (57.7%) developed AKI defined by RIFLE classification. The risk factors for AKI in septic patients were older age, presence of previous CKD, presence of shock, positive blood culture results, previous medication of ACEI/ARB, and lower WBC and platelet counts. The development and severity of AKI was associated with the increased mortality and length of hospital/ICU stay. Additionally, crude 30-day survival decreased as the severity of AKI increased.
AKI is more frequently observed in patients with sepsis and septic shock than in patients with other conditions.
3 The present study showed that among 992 patients with sepsis and septic shock, 573 (57.7%) developed AKI defined by RIFLE classification. These findings are consistent with the previous data. An observational cohort study of 390 patients with septic shock in a single center ICU for about 2 years reported nearly 2 out of 3 patients experiencing AKI.
4 In a recent retrospective multicenter study of 4532 patients with septic shock, a similar percentage of patients (64.4%) developed AKI.
5
In this study, AKI was more frequently accompanied by older age, and male or female predominance was not observed. It is controversial whether these demographic differences actually affect the development of septic AKI. In a previous study conducted in Australia, septic AKI was more prevalent in the older patients.
16 In the present study, multivariable logistic regression analysis showed that sex predilection was not a risk factor for the development of septic AKI. Another study of AKI in septic shock, however, conducted in Germany, showed male dominance in the development of AKI in patients with severe sepsis and septic shock,
5 where older patients were more likely to experience AKI in this study. In contrast, no significant differences were observed in the age and frequency of male sex between the patient groups with or without AKI in a more recent study.
4 Despite some discrepancies among studies, more careful approaches to kidney protection are required in the management of older aged patients with sepsis and septic shock.
We proved that pre-existing CKD is one of the risk factors for the development of AKI, which was also found in previous studies. In a previous study, which enrolled the patients with severe sepsis and septic shock, the frequency of pre-existing CKD was significantly higher among those who developed AKI, although multivariable analysis for the risk factors of septic AKI was not conducted in that study.
7 However, another study, which only analyzed patients with septic shock, noted decreased baseline GFR as an independent risk factor for development of AKI in the population.
5 Our results indicate that a history of CKD is one risk factor among patients with severe sepsis and septic shock, as well as non-severe sepsis.
The pathophysiology of septic AKI, although still not firmly established, is considered different from AKI of other etiologies. It is generally understood that systemic hypotension would deteriorate renal blood flow, which might be a major cause of the renal injury in septic patients. Other studies of septic AKI documented the longer duration of hypotension before hemodynamic resuscitation was associated with higher incidence of AKI.
5,
17 In accordance of these findings, we noted that the presence of shock was one of the risk factors of septic AKI.
High incidence of AKI in patients with sepsis, but without shock, however, cannot be explained with decreased renal blood flow alone. In their experimental animal study, Langenberg, et al.,
10 found AKI in septic shock mainly affected by dysfunction of the renal vascular bed rather than systemic hypotension and renal hypoperfusion. In their study, fluid resuscitation and maintenance of renal perfusion did not prevent the deterioration of GFR.
10 In contrast, recovery from sepsis resulted in the normalization of GFR.
10 This implies the development of AKI involves inflammatory/immunologic mechanisms. Interestingly, in this study, a higher percentage of positive blood culture results were observed in patients with AKI. A similar finding was previously observed.
4 It could be postulated that uncontrolled infection would lead to more intense immunologic responses, resulting in renal dysfunction. The present study showed that gram negative bacteremia was more prevalent in patients with AKI. Gram negative bacilli are well-known sources of the endotoxin, which exerts nephrotoxicity. Our findings further support the notion that the development of septic AKI is not solely affected by hemodynamic instability. Whether the early and adequate administration of antibiotics prevents the development of septic AKI needs to be further elucidated.
Considering the pathogenesis of septic AKI, it is not surprising the use of ACEI/ARB affects renal injury in septic patients.
18,
19 ACEI/ARB are known to decrease the intraglomerular pressure in normal subjects. This action mechanism might be hazardous in sepsis and septic shock, in such conditions where renal blood flow are prone to decrease, resulting in further decrements of intraglomerular pressure and GFR.
An interesting finding is low WBC and platelet counts were associated with septic AKI. Although higher WBC counts were observed in the septic AKI group when compared the patients with septic and nonseptic AKI in a previous study,
16 lower WBC counts were strongly associated with the AKI among septic patients in our study. Moreover, low platelet counts in septic AKI patients are consistently observed in the previous studies on septic AKI.
3,
4,
6 A possible explanation is a more severe inflammatory response might contribute to the development of AKI. It is known that the secretion of pro-inflammatory cytokines, such as IL-1β, TNF-α, and IL-6, is increased in the initial stage of sepsis, followed by activation of anti-inflammatory mechanisms.
20-
22 In a recent study from an experimental sepsis model, the pattern of serum cytokine levels was associated initial leukopenia and prolonged thrombocytopenia.
23 Therefore, AKI in sepsis is not solely affected by hemodynamic changes, lower WBC and platelet counts might indicate more severe immunologic responses subsequently leading to AKI.
Our data clearly suggest the crude 30-day mortality increases as the severity of AKI increases in patients with sepsis and septic shock. It has been elucidated from other previous studies that AKI remains one of the independent risk factors of adverse clinical outcomes even after the adjustment of covariates.
3,
6 Because the development of septic AKI is linked to the increased mortality, patients at a high-risk for septic AKI should be carefully assessed so that nephrotoxic agents or procedures that could be harmful to renal function could be avoided at the initial stage of sepsis management.
There are limitations to our study. First, this study is a retrospective and is potentially susceptible to several forms of bias. Second, our data were collected in a single university hospital. The incidence and severity of diseases might be biased. Third, duration of follow-up was short. We could not assess patients with more severe AKI, such as those who might be classified to loss or ESRD stage according to RIFLE criteria. We also could not investigate the effect of septic AKI on long-term survival. Despite these demerits, our study has some advantages. First, we reviewed a relatively large number of medical records. Second, we revealed various risk factors associated with the development of septic AKI, some of which are of novel. The possible link between gram negative bacteremia and septic AKI was, to our best knowledge, not previously reported. However, the underlying mechanism and specific micro-organism identified by blood culture were not investigated in this study, which remains to be further elucidated.
In conclusion, AKI developed in more than half of patients with sepsis and septic shock. Old age, pre-existing CKD, presence of shock, positive blood culture results, use of ACEI/ARB, and low WBC and platelet counts were associated with an increased risk for the development of septic AKI. The development of septic AKI adversely affected clinical outcomes. Moreover, the severity of AKI was associated with increased short-term mortality.






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download