Abstract
Purpose
The aim of this study was to evaluate long-term oncologic outcomes after concurrent chemoradiation treatment for anal cancer.
Materials and Methods
Between January 1979 and December 2008, the records of 50 consecutive patients with anal cancer and who were treated by chemoradiation or radiation only with a curative intent were retrospectively reviewed. The oncologic outcomes and the risk factors for recurrence were analyzed.
Results
Of the 50 patients, 49 underwent concurrent chemoradiation and one underwent radiation only. After these definitive treatments, 43 (86.0%) achieved a clinical complete response. During the median follow-up of 60 months (range: 2-202 months), the 5-year overall survival, disease-free survival, and locoregional recurrence-free survival were 84.2%, 72.7%, and 69.9%, respectively. Multivariate analysis revealed that the performance status (p=0.031) and a clinical complete response (p=0.039) were the independent predictors for overall survival; lymph node involvement (p=0.031) was the only independent predictor for disease-free survival.
The treatment paradigm for anal cancer has shifted from abdominoperineal resection (APR) with permanent colostomy to combined chemoradiation with sphincter preservation. A recent randomized trial also confirmed chemoradiotherapy to be the standard treatment for anal cancer.1
The total dose of radiation therapy for anal cancer continues to be evaluated. Although the total radiation dose is known to affect local control2,3 the benefit of a high dose over 60 Gy may be doubtful and a high radiation dose may be associated with complications.4,5 Whether or not the addition of inguinal irradiation is beneficial also remains controversial. Some investigators have reported that prophylactic inguinal irradiation for anal cancer shows a low inguinal metastasis rate (<5%),2,6,7 whereas others have suggested that inguinal metastasis is uncommon in anal cancer patients who did not receive routine inguinal irradiation, with inguinal metastasis in only 7.8% of these patients.8
The aim of this study was to evaluate long-term oncologic outcomes after concurrent chemoradiation for anal cancer. Whether a difference of the total radiation dose, as well as the addition of inguinal irradiation, could affect the outcomes was also delineated.
We reviewed the medical records of 55 consecutive patients who were treated with chemoradiation with a curative intent for anal cancer at our institution from January 1979 to December 2008. Patients with distant metastasis (n=2) and who underwent palliative therapy (n=3) were excluded. Ultimately, 50 patients were included. This study was approved by the appropriate institutional review board and informed consent was waived.
Pretreatment evaluation included a physical examination, anoscopy with tumor biopsy, chest X-ray or chest computed tomography (CT), abdomino-pelvic CT, and endorectal ultrasonography or pelvic magnetic resonance imaging (MRI). Clinical tumor staging was established according to the American Joint Committee on Cancer/International Union Against Cancer 7th tumor-node-metastasis (TNM) staging criteria.9 The general condition of the patients was classified using the Eastern Cooperative Oncology Group performance score.10
Using the three or four field techniques, the entire pelvis, including primary tumor and involved node area, were treated with a total of 50.4 Gy (range: 43.2-72.0 Gy) in 28 fractions of 1.8 Gy daily. The median overall treatment time was 45 days (range: 19-80 days). Irradiation of inguinal node areas was performed with a 12 MeV electron beam or 10 MV X-ray. The lateral inguinal nodes were not routinely included in the radiation fields to avoid the injury of the femoral heads, but 13 patients received additional irradiation to the inguinal area due to a positive inguinal node seen on an imaging study (n=3) and the doctor's choice for performing prophylaxis (n=10).
Of the 50 patients, 49 received concurrent chemoradiotherapy. Three chemotherapeutic regimens were used. One involved 5-fluorouracil (FU) and mitomycin [5-FU 1000 mg/m2/day IV from day 1 to day 4, mitomycin 15 mg/m2 IV bolus on day 1, with two cycles of these regimens repeated in weeks 1 and 5 (n=36)]. The second involved 5-FU and cisplatin [5-FU 1000 mg/m2/day IV from day 1 to day 4, cisplatin 25 mg/m2/day IV from day 1 to day 4, with four cycles of these regimens repeated in weeks 1 and 5 (n=12). The third involved 5-FU alone (n=1)].
Upon completion of curative treatment, patients were re-evaluated 8 and 12 weeks later by a digital rectal examination with anoscopy. A clinical complete remission (CR) was defined as clinical, histologic, and/or radiographic evidence of the disappearance of the tumor. Of the 50 patients, 43 (86.0%) achieved a clinical CR. Although 7 patients with a non-CR were considered for salvage APR, only one underwent APR. The remaining 6 patients were closely followed-up without any additional treatment because they had a poor general condition or refused surgery.
Treatment morbidity was graded by Radiation Therapy Oncology Group (RTOG) radiation morbidity scoring criteria.11 Acute and late toxicities were graded on a scale of 1 (most benign) to 5 (toxicity resulting in patient demise).
After completion of curative therapy with chemoradiation, patients were initially followed up at 3-month intervals for 2 years, every 6 months for the next 3 years, and then every 5 years. On a semiannual basis or when there was a suspicion of recurrence, the follow-up examinations included a clinical history, physical examination, anoscopy, chest X-ray or CT, abdomino-pelvic CT or pelvic MRI, and positron emission tomography (PET) scanning if available. Local recurrence was defined as recurrence within the pelvis and regional recurrence included a pelvic or inguinal lymph node recurrence. Systemic recurrence was defined as disease outside of the pelvis. The main patterns of recurrence were recorded as the first site of detectable failure during the follow-up period.
Statistical analyses were as performed using the statistical package SPSS for Windows (version 18.0, SPSS, Chicago, IL, USA). Differences between the 2 groups were analyzed using the χ2 test, Fisher's exact test, and Student's t test, as appropriate. The Kaplan-Meier method was used to analyze survival and the log-rank test was used to assess statistical significance. Only the factors that had a p value <0.20, as determined on univariate analysis, were entered into the Cox's regression model. A value of p<0.05 was considered statistically significant.
The median age of the 50 patients (38 males, 12 females) at presentation was 63.5 years (range: 39-89 years). Tumor histology revealed squamous cell carcinoma in 40 (80.0%) patients, cloacogenic carcinoma in 5 (10.0%) patients, adenocarcinoma in 4 (8.0%) patients, and basal cell carcinoma in one (2.0%) patient. Of these, 13 patients had cT1 tumor, 27 had cT2 tumor, 4 had cT3 tumor, and 6 had cT4 tumor. Thirty two (64.0%) patients were lymph node negative, whereas 18 (36.0%) patients had involved lymph nodes. After chemoradiation, 5 (10.0%) patients experienced acute grade 3 dermatitis and 2 of these patients had a rectovaginal fistula (one patient also had Fournier's gangrene). The patient with Fournier's gangrene was offered salvage APR, and the remaining patients were treated with conservative treatment and they showed favorable results.
Comparative data of the clinicopathologic variables between the patients with <50.4 Gy and those with ≥50.4 Gy are shown in Table 1. The patients who received ≥50.4 Gy had a higher incidence of clinical lymph node involvement (p=0.024). However, there were no significant differences in terms of age, gender, performance status, tumor histology, clinical T stage, clinical N stage, clinical CR, 5-year overall survival (OS), 5-year disease-free survival (DFS), and 5-year locoregional recurrence-free survival (LRFS) (Table 1).
Comparisons of the clinicopathologic variables between the patients without inguinal irradiation and those with irradiation are summarized in Table 2. The patients without inguinal irradiation had a good performance status (p=0.006) and a lower frequency of clinical lymph node involvement (p=0.001). However, there were no significant differences in terms of age, gender, tumor histology, clinical T stage, clinical CR, 5-year OS, 5-year DFS, and 5-year LRFS (Table 2). Thirteen of the 50 patients (26%) who received inguinal irradiation had no recurrence at the inguinal area during the follow-up period, whereas 4 (11.7%) of the 37 patients who did not receive inguinal irradiation at the inguinal region experienced local failure at this area.
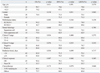
Univariate analysis indicated that the factors associated with 5-year OS were gender, the performance status, and a clinical CR; the only factor associated with DFS and LRFS was the clinical N stage (Table 3). The multivariate analyses revealed that the performance status and a clinical CR were the independent prognostic factors for OS; the clinical N stage was the only independent prognostic variable for both DFS and LRFS, and a clinical CR had a tendency to predict for both DFS and locoregional RFS (Table 4).
During the median follow-up of 60 months (range: 2-202 months), 39 (78%) patients were alive without disease and one was alive with disease, whereas 10 had recurrence of disease (20.0%) and the median time to recurrence among the 10 patients was 18 months (range: 3-38 months). Fourteen of 50 patients had a colostomy due to salvage surgery for a non-CR or for treating a rectovaginal fistula. The 5-year OS, DFS, and LRFS were 84.2%, 72.7%, and 69.9%, respectively. The 5-year OS rates of the patients with a negative clinical N stage and a positive clinical N stage were 85% and 81%, respectively (p=0.076) (Fig. 1A). The DFS curves among the two groups significantly differed (76% vs. 64%, respectively; p=0.035) (Fig. 1B). The 5-year OS rates of the patients with a clinical CR and a non-clinical CR were 92% and 36%, respectively (p=0.007) (Fig. 2A). However, the 5-year DFS curves among the two groups differed (76% vs. 36%, respectively) (Fig. 2B), although the difference did not reach statistical significance (p=0.068).
Patterns of recurrence, treatment modalities, and outcomes are shown in Table 5. Three patients with local recurrence and who underwent APR as salvage treatment are still alive with no evidence of disease. One patient with combined recurrence (a left inguinal lymph node and lung metastasis) underwent an inguinal lymph node dissection following 5-FU/cisplatin chemotherapy and radiation to the inguinal region and she is alive with disease. Among the 3 patients with regional (inguinal area) recurrence, 2 patients who received chemotherapy and additional radiation to the inguinal area have shown no evidence of disease to date. The remaining patient who received chemotherapy only died of disease progression 44 months after post-salvage treatment.
Concurrent chemoradiation has been the standard treatment strategy for anal cancer. The European Organization for Research and Treatment of Cancer study and a large United Kingdom Coordinating Committee on Cancer Research trial showed that chemoradiation with mitomycin and 5-FU was more effective for local control than radiation alone, although there was no significant improvement of OS.12,13 Similarly to other studies,13,14 chemoradiotherapy for anal cancer has been demonstrated to be effective for long-term survival in the current research; the 5-year OS, DFS, LRFS, and colostomy-free survival (CFS) were 84.2%, 72.7%, 69.9%, and 71.3%, respectively.
Mitomycin has been associated with life-threatening hemolytic-uremic syndrome,15 leading to the use of cisplatin as a substitute for mitomycin. The Radiation Therapy Oncology Group conducted a randomized trial (RTOG 98-11) to compare combination therapy (5-FU and mitomycin) versus combination therapy after neoadjuvant 5-FU and cisplatin.16 There were no significant differences in 5-year DFS (60% vs. 54%, p=0.17) and in five-year OS (75% vs. 70%, p=0.10) between the mitomycin group and the cisplatin group. Although higher colostomy rates (19% vs. 10%, p=0.02) were found in the cisplatin group, higher grade 3-4 hematologic toxicity was observed in the mitomycin group (61% vs. 42%, p=0.001).17 We used the other regimen to reduce the toxicity of mitomycin depending on the patient's condition or physician's decision; 12 patients received the 5-FU and cisplatin, one patient refused chemotherapy, and one patient with poor general condition received only 5-FU chemotherapy. The present study also found no differences between patients receiving 5-FU and mitomycin compared to other regimens in OS, DFS, and LRFS.
Intensity-modulated radiation therapy (IMRT) is a novel method of delivering radiation to targets. The IMRT is based on the principle that it can modulate the intensity of the incoming beams of radiation, thus delivering radiation only to the target areas with minimizing damage to normal tissue.18 The RTOG 98-11 study using conventional 3D-radiation therapy showed the rate of acute nonhematologic grade 3-4 toxicity was 74%, and that acute hematologic grade 3-4 toxicity was 61% in the mitomycin-based group and 42% in the cisplatin-based group.16 Whereas several publications have shown the reduced toxicity of IMRT without compromising the local control,19-22 Vieillot, et al.22 reported that 12% patients receiving chemoradiotherapy had grade 4 toxicities, including only hematologic toxicity. The 2-year OS rate, local RFS, and CFS were 89%, 77%, and 85%, respectively. A recent multicenter study also showed that acute grade 3-4 toxicity included hematologic 61%, dermatologic 10%, gastrointestinal 7%, and genitourinary 7% and 2-year local control, OS, and CFS were 95%, 94%, and 90%, respectively.21 However, we performed the conventional 3D-radiation therapy because the Korean public health system does not cover IMRT-related cost. Further studies are needed.
The tumor diameter and lymph node involvement are the most important prognostic factors for survival for patients with anal cancer. A recent randomized study showed that the patients with a tumor diameter exceeding 5 cm and lymph node metastasis were associated with poorer OS and DFS.23 Ajani, et al.24 reported that lymph node positivity had a more unfavorable influence on survival than did the tumor diameter for patients with anal cancer. Touboul, et al.25 suggested that anal cancer patients without lymph node involvement have a 10-year survival rate of 73% compared with 53% for anal cancer patients with nodal involvement. We also found that clinical lymph node positivity was an independent predictor for both DFS and LRFS.
The response to radiation is an independent predictive factor for the survival of anal cancer patients.8,26,27 Deniaud-Alexandre, et al.26 showed that a clinical CR was an independent prognostic predictor for DFS in anal cancer patients, and Schwarz, et al.27 also concluded that a complete metabolic response in anal cancer patients, as determined by the post-therapy PET scan, was the predictor for improvement of PFS. We concur with this notion; we showed that a clinical CR was an independent factor for predicting OS.
We observed that the performance status was an independent predictor for OS.17,28,29 Martenson, et al.17 reported that patients with a good performance status had much better 5-year OS as compared to that of the patients with a poor performance status (74% vs. 55%, respectively; p=0.045). Moreover, Chen, et al.29 showed that the performance status was a significant prognostic factor for OS (p<0.001).
The current findings are limited by the retrospective nature of the analysis and a relatively small number of patients. Nevertheless, our study may allow us to draw valuable conclusions: the performance status and a clinical CR are independent prognostic factors for OS, and patients without lymph node involvement have significantly longer DFS and LRFS than patients with lymph node involvement. Moreover, the addition of irradiation to inguinal area may not significantly be associated with the outcomes. However, long-term randomized trials with larger numbers of patients are needed to confirm this issue.
Figures and Tables
Fig. 1
Survival curves according to clinical lymph node positivity (A) overall survival and (B) disease-free survival.

Fig. 2
Survival curves according to clinical complete remission (A) overall survival and (B) disease-free survival. CR, complete remission.

Table 1
Comparison of the Clinicopathologic Variables between Patients Receiving <50.4 Gy and ≥50.4 Gy
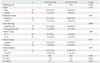
Table 2
Comparison of the Clinicopathologic Variables between the Patients without Inguinal Irradiation and the Patients with Inguinal Irradiation
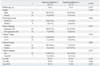
Table 3
Univariate Analyses of the Factors for 5-Year Overall Survival (OS), Disease-Free Survival (DFS), and Locoreginal Recurrence-Free Survival (LRFS)
ACKNOWLEDGEMENTS
This study was supported by a grant (CRI12040-1) from Chonnam National University Hospital Research Institute of Clinical Medicine.
References
1. Northover J, Glynne-Jones R, Sebag-Montefiore D, James R, Meadows H, Wan S, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br J Cancer. 2010. 102:1123–1128.

2. Ferrigno R, Nakamura RA, Dos Santos Novaes PE, Pellizzon AC, Maia MA, Fogarolli RC, et al. Radiochemotherapy in the conservative treatment of anal canal carcinoma: retrospective analysis of results and radiation dose effectiveness. Int J Radiat Oncol Biol Phys. 2005. 61:1136–1142.

3. Huang K, Haas-Kogan D, Weinberg V, Krieg R. Higher radiation dose with a shorter treatment duration improves outcome for locally advanced carcinoma of anal canal. World J Gastroenterol. 2007. 13:895–900.

4. Cantril ST, Green JP, Schall GL, Schaupp WC. Primary radiation therapy in the treatment of anal carcinoma. Int J Radiat Oncol Biol Phys. 1983. 9:1271–1278.

5. Conroy T, Ducreux M, Lemanski C, Francois E, Giovannini M, Cvitkovic F, et al. Treatment intensification by induction chemotherapy (ICT) and radiation dose escalation in locally advanced squamous cell anal canal carcinoma (LAAC): definitive analysis of the intergroup ACCORD 03 trial. J Clin Oncol. 2009. 27:15S. 4033.

6. Cummings BJ, Keane TJ, O'Sullivan B, Wong CS, Catton CN. Epidermoid anal cancer: treatment by radiation alone or by radiation and 5-fluorouracil with and without mitomycin C. Int J Radiat Oncol Biol Phys. 1991. 21:1115–1125.

7. Das P, Bhatia S, Eng C, Ajani JA, Skibber JM, Rodriguez-Bigas MA, et al. Predictors and patterns of recurrence after definitive chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys. 2007. 68:794–800.

8. Gerard JP, Ayzac L, Hun D, Romestaing P, Coquard R, Ardiet JM, et al. Treatment of anal canal carcinoma with high dose radiation therapy and concomitant fluorouracil-cisplatinum. Long-term results in 95 patients. Radiother Oncol. 1998. 46:249–256.

9. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010. 17:1471–1474.

10. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982. 5:649–655.

11. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995. 31:1341–1346.

12. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. Lancet. 1996. 348:1049–1054.
13. Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997. 15:2040–2049.

15. Thomas CR Jr, Stelzer KJ, Douglas JG, Koh WJ, Wood LV, Panicker R. Common emergencies in cancer medicine: infectious and treatment-related syndromes, Part II. J Natl Med Assoc. 1994. 86:839–852.
16. Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB 3rd, Thomas CR Jr, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008. 299:1914–1921.

17. Martenson JA, Lipsitz SR, Lefkopoulou M, Engstrom PF, Dayal YY, Cobau CD, et al. An Eastern Cooperative Oncology Group study. Results of combined modality therapy for patients with anal cancer (E7283). Cancer. 1995. 76:1731–1736.

18. Veldeman L, Madani I, Hulstaert F, De Meerleer G, Mareel M, De Neve W. Evidence behind use of intensity-modulated radiotherapy: a systematic review of comparative clinical studies. Lancet Oncol. 2008. 9:367–375.

19. Salama JK, Mell LK, Schomas DA, Miller RC, Devisetty K, Jani AB, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: a multicenter experience. J Clin Oncol. 2007. 25:4581–4586.

20. Milano MT, Jani AB, Farrey KJ, Rash C, Heimann R, Chmura SJ. Intensity-modulated radiation therapy (IMRT) in the treatment of anal cancer: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2005. 63:354–361.

21. Kachnic LA, Tsai HK, Coen JJ, Blaszkowsky LS, Hartshorn K, Kwak EL, et al. Dose-painted intensity-modulated radiation therapy for anal cancer: a multi-institutional report of acute toxicity and response to therapy. Int J Radiat Oncol Biol Phys. 2012. 82:153–158.

22. Vieillot S, Fenoglietto P, Lemanski C, Moscardo CL, Gourgou S, Dubois JB, et al. IMRT for locally advanced anal cancer: clinical experience of the Montpellier Cancer Center. Radiat Oncol. 2012. 7:45.

24. Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB 3rd, Thomas CR Jr, et al. Prognostic factors derived from a prospective database dictate clinical biology of anal cancer: the intergroup trial (RTOG 98-11). Cancer. 2010. 116:4007–4013.

25. Touboul E, Schlienger M, Buffat L, Lefkopoulos D, Pène F, Parc R, et al. Epidermoid carcinoma of the anal canal. Results of curative-intent radiation therapy in a series of 270 patients. Cancer. 1994. 73:1569–1579.

26. Deniaud-Alexandre E, Touboul E, Tiret E, Sezeur A, Houry S, Gallot D, et al. Results of definitive irradiation in a series of 305 epidermoid carcinomas of the anal canal. Int J Radiat Oncol Biol Phys. 2003. 56:1259–1273.

27. Schwarz JK, Siegel BA, Dehdashti F, Myerson RJ, Fleshman JW, Grigsby PW. Tumor response and survival predicted by post-therapy FDG-PET/CT in anal cancer. Int J Radiat Oncol Biol Phys. 2008. 71:180–186.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download