Abstract
Purpose
Many clinical guidelines recommend apolipoprotein B (apoB) measurement, particularly in subjects with metabolic syndrome or type 2 diabetes. Recently, we developed a new equation to estimate serum apoB (apoBE). We validated the clinical relevance of apoBE and compared the performance of the equation with conventional lipid measurements and direct measurement of apoB.
Materials and Methods
Study subjects were recruited from patients who visited the Health Screening Center at Kangbuk Samsung Hospital between January and December 2009 for routine medical examinations (n=78125). For analysis of coronary calcium score, we recruited study subjects from the same institution between January 2007 and December 2010 (n=16493).
Results
apoBE was significantly correlated with serum high-sensitivity C-reactive level {r=0.18 [95% confidence interval (CI), 0.18–0.19]} in partial correlation analysis adjusted for age, sex, and body mass index. apoBE was associated with a Framingham risk score indicating more than moderate risk (10-year risk ≥10%), the presence of microalbuminuria, and the presence of coronary artery calcium in multivariate logistic regression analysis. These associations were comparable to those of directly-measured serum apoB [odds ratio per 1 SD 3.02 (2.75–3.27) vs. 2.70 (2.42–3.02) for a Framingham risk score indicating more than moderate risk, 1.31 (1.21–1.41) vs. 1.35 (1.25–1.45) for the presence of microalbuminuria, and 1.33 (1.26–1.41) vs. 1.31 (1.23–1.38) for the presence of coronary calcium score respectively]. These findings were also consistently observed in subgroup analysis for subjects with type 2 diabetes.
Even after reaching the low-density lipoprotein (LDL) cholesterol target level through optimal statin treatment in high-risk patients, residual cardiovascular event (CVE) risk persists.1 Some investigators have suggested that apolipoprotein B (apoB) may be superior to LDL as a predictor of cardiovascular disease (CVD).23 In addition to LDL, cholesterol-enriched very low-density lipoprotein (VLDL) and intermediate-density lipoprotein (IDL) particles have been shown to also be atherogenic.4 apoB 100 is also found in remnant cholesterol (VLDL and IDL cholesterol), in addition to LDL. Thus, apoB level may reflect total atherogenic cholesterol particles (LDL, VLDL, and IDL). Measurement of apoB more accurately predicts cardiovascular risk than LDL, especially in atherogenic dyslipidemia, which is characterized by relatively normal LDL in patients with diabetes and metabolic syndrome. Many clinical guidelines recommend apoB as a secondary target.5678 The European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) recommends that apoB should be considered an alternative risk marker, especially in type 2 diabetes, metabolic syndrome, or chronic kidney disease. The American Association of Clinical Endocrinologist (AACE) recommends that apoB be measured in patients with established coronary artery disease, type 2 diabetes, or insulin resistance syndrome. However, serum apoB level is not routinely measured because of the additional cost. Recently, we developed a new equation to estimate serum apoB (apoBE) from total cholesterol (TC), triglyceride (TG), and high-density lipoprotein (HDL) cholesterol levels.9 However, despite the strong approximation with directly-measured apoB, it is still uncertain whether apoBE has clinical relevance in association with cardiovascular surrogate markers or CVD itself. Therefore, the aim of this study was to validate the clinical relevance of apoBE and to compare the performance of the equation with conventional lipid measurements and directly-measured apoB.
A detailed description of the study design has been published previously.9 Briefly, study subjects were recruited from patients who visited the Health Screening Center at Kangbuk Samsung Hospital between January and December 2009 for routine medical examinations (n=78125). In the analysis of coronary calcium score (CCS), we recruited study subjects from the same institution between January 2007 and December 2010 (n=16493).
The study protocol and data analysis were approved by the Institutional Review Board of Kangbuk Samsung Hospital. Since the data did not include any personal information, the Board determined that the study was exempt from the need for informed consent from study participants.
All blood samples were obtained in the morning following an overnight fast of 12 to 14 hours. Serum TC, TG, HDL, and directly-measured LDL levels were determined using an auto-analyzer (Advia 1800, Siemens, Berlin, Germany). Serum apoB and apoA-I concentrations were determined using immunoturbidimetric methods (Advia 2400, auto-analyzer; Siemens), with inter-assay coefficients of variation (CVs) of 2.1–6.1 and 1.8–4.8%, respectively. High-sensitivity C-reactive protein (hs-CRP) was analyzed using particle-enhanced immunonephelometry with a BNIITM System (Dade Behring, Marburg, Germany) with a lower detection limit of 0.175 mg/L. To measure CCS, a 64-slice multi-detector computed tomography scanner (Lightspeed VCT XTe-64 slice, GE Healthcare, Milwaukee, WI, USA) was used. A standard scanning protocol was employed: 32×0.625-mm section collimation, 400-msec rotation time, 120-kV tube voltage, and 31-mAS (310 mA*0.1 sec) tube current under electrocardiographic-gated dose modulation. The Agatston scoring method was used to quantify CCS,10 which was positively skewed with 95% of patients having a zero value. Therefore, coronary calcification was defined as the presence of any calcium (CCS>0). A single morning voided urine sample at baseline was used to measure the urinary albumin creatinine ratio (UACR) (µg mg-1). The urinary albumin concentration was determined with immune-radiometry (Radio-immunological competition assay, Immunotech Co., Prague, Czech Republic), and urinary creatinine concentration was measured using the modified Jaffe method. The CVs were intra-assay 1–6% and inter-assay <10% for urine albumin and urine creatinine assays, respectively. The presence of albuminuria was defined as a UACR greater than 30 µg mg-1.
Estimation of LDL was calculated using the Friedewald formula: Calculated LDL=TC-HDL-TG/5.11 Framingham risk score was calculated using the most recent version of the scale. Ten-year risk of a CVD event with a Framingham risk score ≥10% was classified as “more than moderate risk.”12
apoBE was calculated using the formula developed from our previous model 2:9
apoBE=0.65×TC-0.59×HDL+0.01×TG, TG≤270,
apoBE=25.6+0.58×TC-0.38×HDL-0.06×TG, TG>270.9
Spearman correlation coefficients between serum hs-CRP and apoBE were calculated using partial correlation analysis. These correlations were adjusted by age, sex, and body mass index (BMI). To assess the associations with cardiovascular surrogate markers, apoBE, and lipid parameters, we performed a multivariate logistic regression analysis using R version 2.14.2 (http://www.r-project.org). p<0.05 were considered statistically significant.
Baseline characteristics of study participants were showed in Table 1. Table 2 shows the association between the apoBE and hs-CRP. apoBE was significantly correlated with serum hs-CRP level {r=0.18 [95% confidence interval (CI), 0.18–0.19]} after adjustment for age, sex, and BMI, and this association was stronger than that of estimated LDL from the Friedewald equation [r=0.11 (95% CI, 0.10–0.12)] or directly-measured LDL [r=0.14 (95% CI, 0.13–0.14)]. However, directly-measured apoB showed a stronger association with serum hs-CRP than estimated apoB [0.22 (95% CI, 0.21–0.22) vs. 0.18 (95% CI, 0.18–0.19)].
Next, we determined whether apoBE was more associated with a Framingham risk score indicating more than moderate risk (10-year risk ≥10%), microalbuminuria, and coronary artery calcium (Table 3) through multivariate binary logistic regression analysis. apoBE was associated with Framingham risk score more than moderate risk, presence of microalbuminuria, and presence of coronary artery calcium [odds ratio (OR) per 1 standard deviation (SD); 3.02 (95% CI, 2.75–3.27), 1.31 (1.21–1.41), and 1.33 (1.26–1.41), respectively]. In addition, the association was stronger than those of estimated LDL [OR per 1 SD 3.02 (2.75–3.27) vs. 1.98 (1.82–2.16) for Framingham risk score indicating more than moderate risk, 1.31 (1.21–1.41) vs. 1.04 (0.99–1.13) for the presence of microalbuminuria, 1.33 (1.26–1.41) vs. 1.21 (1.14–1.27) for the presence of CCS, respectively]. The association was comparable to those of directly-measured serum apoB [OR ratio per 1 SD 3.02 (2.75–3.27) vs. 2.70 (2.42–3.02) for Framingham risk score indicating more than moderate risk, 1.31 (1.21–1.41) vs. 1.35 (1.25–1.45) for the presence of microalbuminuria, 1.33 (1.26–1.41) vs. 1.31 (1.23–1.38) for the presence of CCS, respectively].
These findings were also consistently observed in subgroup analysis for subjects with type 2 diabetes. apoBE was more highly correlated with hs-CRP [r=0.226 (95% CI, 0.207–0.245)] than with LDL estimated by the Friedewald equation [r=0.119 (95% CI, 0.098–0.139)] or directly-measured LDL [r=0.164 (95% CI, 0.145–0.185)] and was comparable with directly-measured apoB [r= 0.258 (95% CI, 0.283–0.323)] (Table 4). apoBE was strongly associated with a Framingham risk score indicating more than moderate risk and the presence of microalbuminuria [OR ratio per 1 SD 3.01 (2.44–3.72) and 1.30 (1.13–1.50), respectively]. However, apoBE was not associated with the presence of coronary artery calcium [OR per 1 SD 1.06 (0.89–1.27)] (Table 5).
In this study, we compared apoBE with directly-measured apoB, estimated LDL, and directly-measured LDL to investigate their associations with CV surrogate markers in order to validate a previously reported apoBE equation. apoBE had a similar association with directly measured apoB for CV surrogate markers. apoBE had a stronger association with CV surrogate markers than estimated or directly-measured LDL. We validated our equation in three ways. First, we compared the association of estimated apoB with CV surrogate markers to that of LDL and directly-measured apoB in this study. Second, we investigated whether estimated apoB could predict CVEs in a low CV risk group with community cohort (PMID 27310947).13 Third, we are currently investigating whether estimated apoB can predict CVEs in a high CV risk group [Treating to New Targets (TNT)/Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) study data].
Chylomicrons, VLDL, and IDL in addition to LDL are also atherogenic in several studies.141516 Remnant cholesterol, like LDL, penetrates to intima of the arterial wall. This causes inflammation of the arterial wall and consequent atherosclerosis. In clinical studies, remnant cholesterol was shown to be a predictor of ischemic heart disease.4 Apolipoprotein is the main surface protein found on all beta-lipoproteins. Since there is a single molecule of apoB on each of these apolipoproteins, the apoB level reflects total atherogenic lipid particle numbers, especially in atherogenic dyslipidemia, characterized by relatively normal cholesterol level with increased small density LDL particles numbers. Accordingly, it has been reported that apoB level can be used to predict cardiovascular events.
hs-CRP, microalbuminuria, Framingham score, and CCS are surrogate markers of CVEs.17181920 In this study, apoBE was correlated with these surrogate markers. Furthermore, these associations were directly comparable to measured apoB, except that of hs-CRP. apoBE had stronger associations with these surrogate markers than with LDL. These findings are observed not only in apparently healthy people, but also in subjects with type 2 diabetes, which is characterized by atherogenic lipid profiles. In some studies, TG and LDL have been reported as risk factors for microalbuminuria.2122 In our study, apoB and apoBE were more potent risk factors for microalbuminuria than estimated or directly-measured LDL. Previous studies did not evaluate the association between apoB and microalbuminuria.
Prenner, et al.23 reported that VLDL is associated with coronary artery calcification in type 2 diabetes.24 Because apoB is the primary apolipoprotein of VLDL, this study was consistent with our findings. And the Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration group found a causal association between TG-mediated pathways and coronary heart disease through a genetic variant study that regulated TG concentration.
The Friedewald equation has been widely used for estimation of LDL. The Friedewald equation and our equation use the same variables (TC, LDL, and TG) and are both linear equations. However, the results of our equation (apoBE) were more closely associated with cardiovascular surrogate markers than were the results of the Friedewald equation (estimated LDL). Moreover, while the Friedewald equation cannot be applied when TG is high, the equation that we proposed can be used in such a situation.
Two other equations have been developed to estimate apoB from routine lipid profiles.2526 While we use three conventional lipid variables (TC, HDL, and TG) for estimation of apoB, similar to the Friedewald equation, two other groups use two variables for this estimation. Cho, et al.25 used directly-measured LDL and TG, and Hermans, et al.26 used TC and HDL. However, those two studies did not demonstrate clinical relevance. Additionally, the sample size of the study conducted by Hermans, et al.26 (n=45) was not large, and Hermans, et al.'s equation was developed from only type 2 diabetes patients. We verified the clinical relevance of our equation in a general population and a population with type 2 diabetes.
Currently, LDL is not routinely directly measured in national health screening programs due to cost; instead, it is measured in those with TG levels higher than 400 mg/dL. LDL can be calculated from TC, TG, and HDL. Our equation to estimate apoB uses the same variables as the Friedewald equation (TC, TG, and HDL). Currently, several guidelines recommend apoB measurement, particularly in metabolic syndrome and type 2 diabetes patients. A considerable proportion of national health screening program participants exhibit indications for apoB measurement. If we could co-automatically calculate apoBE when automatically calculating LDL, it would provide useful additional information. apoB is a secondary target in treatment of dyslipidemia. Estimating apoB using conventional lipid profiles would be cost-saving and provide additional value, especially in atherogenic dyslipidemia patients.
The present study has several limitations. First, because it was a cross-sectional study, we could not infer causal relationships. Second, subjects were chosen from a population of patients that received routine medical examinations and were likely not representative of the general population. Our study subjects also did not represent the population for which apoB measurement is indicated, specifically those with metabolic syndrome, diabetes, or at high risk for CVEs. Fourth, CCS was obtained from a different study population than that from which other cardiovascular surrogate markers (hs-CRP, Framingham risk score, and urine microalbumin) were obtained, because a very small number of subjects underwent CCS measurement in the same year.
In conclusion, apoBE was associated with hs-CRP, Framingham risk score indicating more than moderate risk, and coronary risk calcium score. The association between these surrogate markers and apoBE was comparable to those of directly-measured apoB. apoBE had stronger associations with cardiovascular surrogate markers than did estimated or directly-measured LDL. A longitudinal study of the relationships between CVEs and apoBE is needed.
Figures and Tables
Table 1
Baseline Characteristics of the Study Participants

Table 2
Correlations between Lipid Measurement and hs-CRP

Table 3
Associations between Lipid Measurements and Cardiovascular Surrogate Markers in Multivariate Logistic Regression Analysis

Table 4
Correlations between Lipid Measurements and Cardiovascular Disease Surrogate Markers in Patients with Diabetes
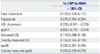
Table 5
Associations between Lipid Measurements and Cardiovascular Disease Surrogate Markers in Multivariate Logistic Regression Analysis for Patients with Diabetes

ACKNOWLEDGEMENTS
This work was supported by a Samsung Biomedical Research Institute grant to Cheol-Young Park and the research program of Dongguk University-Seoul to Hong-Yup Ahn.
References
1. Hausenloy DJ, Yellon DM. Targeting residual cardiovascular risk: raising high-density lipoprotein cholesterol levels. Heart. 2008; 94:706–714.

2. Shai I, Rimm EB, Hankinson SE, Curhan G, Manson JE, Rifai N, et al. Multivariate assessment of lipid parameters as predictors of coronary heart disease among postmenopausal women: potential implications for clinical guidelines. Circulation. 2004; 110:2824–2830.

3. Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005; 294:326–333.

4. Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013; 61:427–436.

5. Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, et al. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008; 51:1512–1524.
6. Catapano AL, Reiner Z, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis. 2011; 217:3–46.
7. Jellinger PS, Smith DA, Mehta AE, Ganda O, Handelsman Y, Rodbard HW, et al. American Association of Clinical Endocrinologists’ guidelines for management of dyslipidemia and prevention of atherosclerosis. Endocr Pract. 2012; 18:Suppl 1. 1–78.

8. Ko SH, Kim SR, Kim DJ, Oh SJ, Lee HJ, Shim KH, et al. 2011 clinical practice guidelines for type 2 diabetes in Korea. Diabetes Metab J. 2011; 35:431–436.

9. Hwang YC, Ahn HY, Lee WJ, Park CY, Park SW. An equation to estimate the concentration of serum apolipoprotein B. PLoS One. 2012; 7:e51607.

10. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990; 15:827–832.

11. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499–502.

12. D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008; 117:743–753.

13. Hwang YC, Park CY, Ahn HY, Cho NH. Prediction of future development of cardiovascular disease with an equation to estimate apolipoprotein B: a community-based cohort study. Medicine (Baltimore). 2016; 95:e3644.
14. Jørgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjærg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013; 34:1826–1833.

15. Varbo A, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013; 128:1298–1309.

16. Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res. 2009; 50:204–213.

17. Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998; 98:731–733.

18. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000; 342:836–843.

19. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001; 286:421–426.

20. Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010; 303:1610–1616.

21. Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. UKPDS Study Group. Risk factors for renal dysfunction in type 2 diabetes: UK Prospective Diabetes Study 74. Diabetes. 2006; 55:1832–1839.
22. Tseng CH. Apolipoprotein B is an independent risk factor for microalbuminuria in Taiwanese patients with type 2 diabetes. Diabetes Care. 2003; 26:2965–2966.

23. Prenner SB, Mulvey CK, Ferguson JF, Rickels MR, Bhatt AB, Reilly MP. Very low density lipoprotein cholesterol associates with coronary artery calcification in type 2 diabetes beyond circulating levels of triglycerides. Atherosclerosis. 2014; 236:244–250.

24. Triglyceride Coronary, Sarwar N, Sandhu MS, Ricketts SL, Butterworth AS, Di Angelantonio E, et al. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010; 375:1634–1639.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download