1. Angiolillo DJ. The evolution of antiplatelet therapy in the treatment of acute coronary syndromes: from aspirin to the present day. Drugs. 2012; 72:2087–2116.

2. Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015; 12:30–47.

3. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009; 361:1045–1057.

4. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007; 357:2001–2015.

5. Noh Y, Lee J, Shin S, Lim HS, Bae SK, Oh E, et al. Antiplatelet therapy of cilostazol or sarpogrelate with aspirin and clopidogrel after percutaneous coronary intervention: a retrospective cohort study using the Korean National Health Insurance Claim Database. PLoS One. 2016; 11:e0150475.

6. Chen KY, Rha SW, Li YJ, Poddar KL, Jin Z, Minami Y, et al. Triple versus dual antiplatelet therapy in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation. 2009; 119:3207–3214.

7. Saini HK, Takeda N, Goyal RK, Kumamoto H, Arneja AS, Dhalla NS. Therapeutic potentials of sarpogrelate in cardiovascular disease. Cardiovasc Drug Rev. 2004; 22:27–54.

8. Miyazaki M, Higashi Y, Goto C, Chayama K, Yoshizumi M, Sanada H, et al. Sarpogrelate hydrochloride, a selective 5-HT2A antagonist, improves vascular function in patients with peripheral arterial disease. J Cardiovasc Pharmacol. 2007; 49:221–227.

9. Satomura K, Takase B, Hamabe A, Ashida K, Hosaka H, Ohsuzu F, et al. Sarpogrelate, a specific 5HT2-receptor antagonist, improves the coronary microcirculation in coronary artery disease. Clin Cardiol. 2002; 25:28–32.

10. Shikata C, Nemoto M, Ebisawa T, Nishiyama A, Takeda N. Effect of sarpogrelate on cardiovascular disorders. Exp Clin Cardiol. 2011; 16:75–76.
11. Schröder R, Dissmann R, Brüggemann T, Wegscheider K, Linderer T, Tebbe U, et al. Extent of early ST segment elevation resolution: a simple but strong predictor of outcome in patients with acute myocardial infarction. J Am Coll Cardiol. 1994; 24:384–391.

12. Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr. 2004; 17:630–633.

13. GUSTO investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993; 329:673–682.
14. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007; 115:2344–2351.
15. Stone GW, Webb J, Cox DA, Brodie BR, Qureshi M, Kalynych A, et al. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: a randomized controlled trial. JAMA. 2005; 293:1063–1072.

16. Grines CL, Bonow RO, Casey DE Jr, Gardner TJ, Lockhart PB, Moliterno DJ, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Circulation. 2007; 115:813–818.

17. Snoep JD, Hovens MM, Eikenboom JC, van der Bom JG, Huisman MV. Association of laboratory-defined aspirin resistance with a higher risk of recurrent cardiovascular events: a systematic review and meta-analysis. Arch Intern Med. 2007; 167:1593–1599.

18. Snoep JD, Hovens MM, Eikenboom JC, van der Bom JG, Jukema JW, Huisman MV. Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J. 2007; 154:221–231.

19. Lev EI, Patel RT, Maresh KJ, Guthikonda S, Granada J, DeLao T, et al. Aspirin and clopidogrel drug response in patients undergoing percutaneous coronary intervention: the role of dual drug resistance. J Am Coll Cardiol. 2006; 47:27–33.

20. Müller I, Seyfarth M, Rüdiger S, Wolf B, Pogatsa-Murray G, Schömig A, et al. Effect of a high loading dose of clopidogrel on platelet function in patients undergoing coronary stent placement. Heart. 2001; 85:92–93.

21. Gawaz M, Neumann FJ, Ott I, Schiessler A, Schömig A. Platelet function in acute myocardial infarction treated with direct angioplasty. Circulation. 1996; 93:229–237.

22. Storey RF, Becker RC, Harrington RA, Husted S, James SK, Cools F, et al. Characterization of dyspnoea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes. Eur Heart J. 2011; 32:2945–2953.

23. Scirica BM, Cannon CP, Emanuelsson H, Michelson EL, Harrington RA, Husted S, et al. The incidence of bradyarrhythmias and clinical bradyarrhythmic events in patients with acute coronary syndromes treated with ticagrelor or clopidogrel in the PLATO (Platelet Inhibition and Patient Outcomes) trial: results of the continuous electrocardiographic assessment substudy. J Am Coll Cardiol. 2011; 57:1908–1916.

24. Roe MT, Armstrong PW, Fox KA, White HD, Prabhakaran D, Goodman SG, et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012; 367:1297–1309.

25. Ashton JH, Benedict CR, Fitzgerald C, Raheja S, Taylor A, Campbell WB, et al. Serotonin as a mediator of cyclic flow variations in stenosed canine coronary arteries. Circulation. 1986; 73:572–578.

26. Bush LR, Campbell WB, Kern K, Tilton GD, Apprill P, Ashton J, et al. The effects of alpha 2-adrenergic and serotonergic receptor antagonists on cyclic blood flow alterations in stenosed canine coronary arteries. Circ Res. 1984; 55:642–652.

27. Eidt JF, Ashton J, Golino P, McNatt J, Buja LM, Willerson JT. Thromboxane A2 and serotonin mediate coronary blood flow reductions in unsedated dogs. Am J Physiol. 1989; 257(3 Pt 2):H873–H882.

28. Faxon DP, Weber VJ, Haudenschild C, Gottsman SB, McGovern WA, Ryan TJ. Acute effects of transluminal angioplasty in three experimental models of atherosclerosis. Arteriosclerosis. 1982; 2:125–133.

29. Steele PM, Chesebro JH, Stanson AW, Holmes DR Jr, Dewanjee MK, Badimon L, et al. Balloon angioplasty. Natural history of the pathophysiological response to injury in a pig model. Circ Res. 1985; 57:105–112.

30. Lam JY, Chesebro JH, Steele PM, Dewanjee MK, Badimon L, Fuster V. Deep arterial injury during experimental angioplasty: relation to a positive indium-111-labeled platelet scintigram, quantitative platelet deposition and mural thrombosis. J Am Coll Cardiol. 1986; 8:1380–1386.

31. Badimon L, Badimon JJ, Turitto VT, Vallabhajosula S, Fuster V. Platelet thrombus formation on collagen type I. A model of deep vessel injury. Influence of blood rheology, von Willebrand factor, and blood coagulation. Circulation. 1988; 78:1431–1442.

32. Pope CF, Ezekowitz MD, Smith EO, Rapoport S, Glickman M, Sostman HD, et al. Detection of platelet deposition at the site of peripheral balloon angioplasty using indium-111 platelet scintigraphy. Am J Cardiol. 1985; 55:495–497.

33. Heras M, Chesebro JH, Penny WJ, Bailey KR, Badimon L, Fuster V. Effects of thrombin inhibition on the development of acute platelet-thrombus deposition during angioplasty in pigs. Heparin versus recombinant hirudin, a specific thrombin inhibitor. Circulation. 1989; 79:657–665.

34. Leosco D, Fineschi M, Pierli C, Fiaschi A, Ferrara N, Bianco S, et al. Intracoronary serotonin release after high-pressure coronary stenting. Am J Cardiol. 1999; 84:1317–1322.

35. Hara H, Osakabe M, Kitajima A, Tamao Y, Kikumoto R. MCI-9042, a new antiplatelet agent is a selective S2-serotonergic receptor antagonist. Thromb Haemost. 1991; 65:415–420.

36. Tanaka T, Fujita M, Nakae I, Tamaki S, Hasegawa K, Kihara Y, et al. Improvement of exercise capacity by sarpogrelate as a result of augmented collateral circulation in patients with effort angina. J Am Coll Cardiol. 1998; 32:1982–1986.

37. Grover GJ, Sargent CA, Dzwonczyk S, Normandin DE, Antonaccio MJ. Protective effect of serotonin (5-HT2) receptor antagonists in ischemic rat hearts. J Cardiovasc Pharmacol. 1993; 22:664–672.

38. Shimizu Y, Minatoguchi S, Hashimoto K, Uno Y, Arai M, Wang N, et al. The role of serotonin in ischemic cellular damage and the infarct size-reducing effect of sarpogrelate, a 5-hydroxytryptamine-2 receptor blocker, in rabbit hearts. J Am Coll Cardiol. 2002; 40:1347–1355.

39. Horibe E, Nishigaki K, Minatoguchi S, Fujiwara H. Sarpogrelate, a 5-HT2 receptor blocker, may have a preconditioning-like effect in patients with coronary artery disease. Circ J. 2004; 68:68–72.


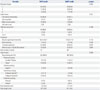
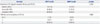







 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download