Abstract
Alveolar capillary dysplasia with misalignment of pulmonary veins (ACD/MPV) is an autosomal dominant, fatal developmental disorder of the lungs, with a mortality rate of about 100%. ACD/MPV is caused by mutations in FOXF1. Herein, we describe a newborn boy with ACD/MPV carrying a novel pathogenic variant of FOXF1. The patient developed respiratory distress and severe pulmonary hypertension on the first day of life. Despite aggressive cardiorespiratory management, including veno-venous extracorporeal membrane oxygenation, his condition deteriorated rapidly, and he died within the first month of his life. Lung histology showed the characteristic features of ACD/MPV at autopsy. Sequence analysis of FOXF1 from genomic DNA obtained from autopsied lung tissue revealed that the patient was heterozygous for a novel missense variant (c.305T>C; p.Leu102Pro). Further analysis of both parents confirmed the de novo occurrence of the variant. To the best of our knowledge, this is the first report of genetically confirmed ACD/MPV in Korea.
Alveolar capillary dysplasia with misalignment of pulmonary veins (ACD/MPV; MIM 265380) is a rare and lethal developmental disorder of the lungs that manifests in newborns as severe respiratory distress, pulmonary hypertension, and characteristic histologic features.1 Within the first few days of life, affected neonates develop respiratory distress and severe pulmonary hypertension unresponsive to treatment, and they die of respiratory failure within the first month of life.2 Fifty to 80% of patients with ACD/MPV have additional non-pulmonary anomalies.1
ACD/MPV can be caused by mutations in FOXF1.2 About 40% of individuals diagnosed with ACD/MPV have mutations in FOXF1.1 Although most cases of ACD/MPV are sporadic, about 10% of cases are familial.34
Here, we present a Korean newborn who died from irreversible respiratory failure within the first month of life. At autopsy, lung histology displayed the characteristic features of ACD/MPV, and we identified a novel pathogenic variant in FOXF1.
The patient was a male newborn of gestational age of 41+1 weeks with no significant family history. Prenatal ultrasound did not show any anomaly, except a 4-cm hypoechoic cyst in the abdomen. He was born at a weight of 3.16 kg via vaginal delivery, and Apgar scores were 8 and 9 at 1 minute and 5 minutes, respectively. During the first hours of life, he developed tachypnea and desaturation and was admitted to the neonatal intensive care unit. A chest radiograph revealed bilateral pneumothorax (Fig. 1). The patient was intubated, and bilateral chest tubes were placed. When a discrepancy in the preductal and postductal saturations became greater than 10%, persistent pulmonary hypertension of the newborn was suspected. Inhaled nitric oxide (NO) was applied; however, hypoxemia was repeated and aggravated. Cardiopulmonary resuscitation was performed, and veno-venous extracorporeal membrane oxygenation (ECMO) was started on day 2. An echocardiogram showed an enlarged right heart and bulging of the interatrial and interventricular septa toward the left heart. He had recurrent bilious vomiting; the diagnosis of malrotation without midgut volvulus was made, and lysis of Ladd's band was performed on day 5. Despite aggressive cardiorespiratory management, including inotropic support, inhaled NO, and nebulized Iloprost, the patient could not be weaned from ECMO, and he died on the 31st day of life. Permission for complete autopsy examination was obtained.
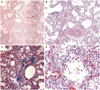
On gross examination, both lungs were solid. The heart showed right ventricular hypertrophy, patent foramen ovale, and constricted ductus arteriosus. The liver, which had symmetric bilateral lobes, was enlarged. Intestinal malrotation was present. The gallbladder and appendix were absent. Histologic findings in the lungs were consistent with the diagnosis of ACD/MPV (Fig. 2).
Genomic DNA was extracted from lung tissue frozen during the autopsy, and all two coding exons were amplified by PCR using primers that we designed. PCR products were sequenced on the ABI 3730xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) using BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems). Sequences were compared with a reference sequence of FOXF1 (NM_001451.2).
The patient was heterozygous for a novel missense variant of FOXF1 (c.305T>C; p.Leu102Pro) in exon 1 (Fig. 3). The p.Leu102Pro variant was absent from the dbSNP (http://www.ncbi.nlm.nih.gov/snp/), Exome Sequencing Project database (http://evs.gs.washington.edu/EVS/), and in-house variants database from 400 Korean control exomes. Bioinformatic analyses revealed that the affected residue Leu102 is strictly conserved from zebrafish to humans and predicted that p.Leu102Pro would be deleterious by means of SIFT (http://sift.bii.a-star.edu.sg/index.html) and PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/). Neither of his parents carried the variant, indicating a de novo occurrence. We regarded that as pathogenic variant according to the standards and guidelines of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology.5
Although atypical late presenters and long-term survivors have been reported,67 ACD/MPV is a fatal disorder that should be considered in neonates with idiopathic persistent pulmonary hypertension whose symptoms recur after weaning from ECMO.1 Extrapulmonary findings are frequently observed, including gastrointestinal, cardiac, and renal anomalies, as well as disruption of right-left asymmetry.18 Among those anomalies, intestinal malrotation is the most frequent manifestation.2
In this report, we described a Korean newborn with ACD/MPV carrying a novel de novo variant in FOXF1. He showed severe pulmonary hypertension refractory to treatment, accompanied by intestinal malrotation. Based on his clinical manifestations, we suspected ACD/MPV and performed an autopsy with sequence analysis of FOXF1.
FOXF1 belongs to the FOX family of transcription factors, which are characterized by a conserved DNA-binding domain (DBD).4 About 60% of all reported FOXF1 mutations are located within the DBD of FOXF1 protein.4 The missense variant identified in our study was also located on the highly conserved DBD, confirming that this domain plays a crucial role in the proper functioning of this important transcription factor.
FOXF1 is known for its crucial role in lung morphogenesis and intrinsic pulmonary vascular development.9 In an experiment involving Foxf1 knockout mice, loss of Foxf1 resulted in death in utero due to pulmonary vascular abnormalities.10 Half of the mutant mice harboring a heterozygous Foxf1 mutation died from pulmonary hypoplasia.10 Recently, Ren, et al.11 reported that FOXF1 is required for the formation of embryonic vasculature to regulate endothelial genes and vascular endothelial growth factor signaling.
Diagnosis of ACD/MPV might be challenging for several reasons. First, definitive diagnosis currently depends on pathologic features of lung tissue on autopsy or ante mortem lung biopsy. However, neither of these procedures is pursued in the clinical setting of a critically ill newborn.1 Second, clinical heterogeneity of ACD/MPV might allow the lung defect to go undetected.712 Third, a lack of specific clinical knowledge among attending physicians could also contribute to underdiagnosis. Therefore, the diagnostic accuracy could be improved by the use of FOXF1 genetic testing, as shown in our case.
Altogether, we identified a novel pathogenic variant of FOXF1 possibly causing ACD/MPV. To the best of our knowledge, this is the first clinicopathologically and genetically confirmed case of ACD/MPV in the Korean population. Our findings also highlight the importance of FOXF1 testing for diagnosis in neonates with refractory pulmonary hypertension of unknown origin.
Figures and Tables
Fig. 1
(A) Chest radiograph at 2 hours of life showing bilateral pneumothorax. (B) After C-tube insertion.
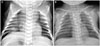
Fig. 2
Histopathologic findings of the deceased newborn with alveolar capillary dysplasia with misalignment of pulmonary veins. (A and B) Malpositioned pulmonary vein branches within the same adventitial sheath with adjacent pulmonary arteries. (C) Masson's trichrome stain highlighting the same adventitial sheath. (D) Improperly positioned capillaries within the walls of the alveoli away from the alveolar epithelium.
References
1. Bishop NB, Stankiewicz P, Steinhorn RH. Alveolar capillary dysplasia. Am J Respir Crit Care Med. 2011; 184:172–179.

2. Stankiewicz P, Sen P, Bhatt SS, Storer M, Xia Z, Bejjani BA, et al. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet. 2009; 84:780–791.
3. Sen P, Gerychova R, Janku P, Jezova M, Valaskova I, Navarro C, et al. A familial case of alveolar capillary dysplasia with misalignment of pulmonary veins supports paternal imprinting of FOXF1 in human. Eur J Hum Genet. 2013; 21:474–477.

4. Sen P, Yang Y, Navarro C, Silva I, Szafranski P, Kolodziejska KE, et al. Novel FOXF1 mutations in sporadic and familial cases of alveolar capillary dysplasia with misaligned pulmonary veins imply a role for its DNA binding domain. Hum Mutat. 2013; 34:801–811.
5. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–424.

6. Szafranski P, Dharmadhikari AV, Wambach JA, Towe CT, White FV, Grady RM, et al. Two deletions overlapping a distant FOXF1 enhancer unravel the role of lncRNA LINC01081 in etiology of alveolar capillary dysplasia with misalignment of pulmonary veins. Am J Med Genet A. 2014; 164A:2013–2019.
7. Ito Y, Akimoto T, Cho K, Yamada M, Tanino M, Dobata T, et al. A late presenter and long-term survivor of alveolar capillary dysplasia with misalignment of the pulmonary veins. Eur J Pediatr. 2015; 174:1123–1126.

8. Miranda J, Rocha G, Soares P, Morgado H, Baptista MJ, Azevedo I, et al. A novel mutation in FOXF1 gene associated with alveolar capillary dysplasia with misalignment of pulmonary veins, intestinal malrotation and annular pancreas. Neonatology. 2013; 103:241–245.

9. Mahlapuu M, Enerbäck S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development. 2001; 128:2397–2406.

10. Kalinichenko VV, Lim L, Stolz DB, Shin B, Rausa FM, Clark J, et al. Defects in pulmonary vasculature and perinatal lung hemorrhage in mice heterozygous null for the Forkhead Box f1 transcription factor. Dev Biol. 2001; 235:489–506.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download