Abstract
Autoimmune retinopathy (AIR) is an immune-mediated retinopathy, resulting from an immunologic process caused by the aberrant recognition of retinal antigens as autoantigens. The diagnosis of AIR involves the detection of antiretinal antibodies with concurrent clinical and electrophysiological evidence of retinopathy. A 40-year-old patient presented with progressive loss of bilateral vision over several months. A fundus examination was unremarkable. Spectral domain optical coherence tomography revealed a blurred photoreceptor ellipsoid zone at the subfoveal region in both eyes with more prominent disruption in the left eye. Fullfield electroretinography (ERG) showed relatively normal rod and cone responses in the right eye, and decreased photopic bwaves with minimal attenuation of a-waves in the left eye. Multifocal ERG demonstrated slightly reduced amplitude of the inner segment ring in the right eye and decreased amplitudes and delayed latencies of all modalities in the left eye. The patient was suspected to have AIR and it was supported by positive Western blots for 23-kDa protein, enolase (46-kDa), aldolase (40-kDa), 62-kDa and 78-kDa proteins and by immunohistochemical staining of human retinal bipolar and ganglion cells. Despite the immunosuppressive treatment, the destruction of the retinal photoreceptors progressed, and immunosuppressive interventions produced very little visual improvement. We report on what is, to the best of our knowledge, the very first case of serologically confirmed nonparaneoplastic AIR in Korea.
Autoimmune retinopathy (AIR) is an immune-mediated retinopathy caused by circulating antiretinal antibodies. It can be categorized as paraneoplastic AIR including cancer-associated retinopathy (CAR), melanoma-associated retinopathy (MAR), possibly bilateral diffuse uveal melanocytic proliferation (BDUMP), and nonparaneoplastic AIR.1 Although the presence of antiretinal antibodies is diagnostic for AIR,2 the diagnosis is ultimately clinical, supported by bilateral, often asymmetric, progressive vision loss over several weeks to months3 and with dysfunction of photoreceptors or bipolar cells, resulting in electroretinographic abnormalities.4 Here, we report the very first case of serologically confirmed nonparaneoplastic AIR in Korea.
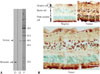
A 40-year-old man visited our clinic in January 2014, having had progressive central vision loss in his left eye (OS) for 1 year and similar symptoms starting in his right eye (OD) 3 months prior. He did not have any significant medical history including any autoimmune diseases and was not on any medication. Also, his ophthalmic history was unremarkable without history of undergoing ocular surgery or any significant ocular diseases. The initial best-corrected visual acuity (BCVA) by a Snellen chart was 20/40 in both eyes (OU). The OS was unable to correctly identify any of the Ishihara color plates. A fundus examination was unremarkable (Fig. 1A). Spectral domain-optical coherence tomography (SD-OCT) revealed a blurred ellipsoid zone at the subfoveal region in OU with more prominent disruption in OS (Fig. 1B). A 30-2 Humphrey visual field (HVF) confirmed central field loss in OS (Fig. 1D). Full-field ERG showed relatively normal rod and cone responses in OD, but slightly decreased photopic b-waves with minimal attenuation of a-waves in OS (Fig. 2A). Multifocal ERG (mfERG) showed slightly reduced amplitude of the inner segment ring in OD and decreased amplitudes and delayed latencies of all modalities in OS (Fig. 2B). Complete medical and oncologic evaluations were performed by a medical internist; however, no underlying abnormalities were found. RP1L1 gene analysis was performed to rule out occult macular dystrophy, but no mutation was detected. We suspected AIR and sent serum samples to the Oregon Health and Science University (http://www.ohsu.edu/xd/health/services/casey-eye/diagnostic-services/ocular-immunology-lab/services.cfm) for examination for antiretinal antibodies. A Western blot was positive for 23-kDa protein, enolase (46-kDa), aldolase (40-kDa), 62-kDa and 78-kDa protein (Fig. 3A). Moderate immunohistochemical staining of some human retinal bipolar and ganglion cells was observed (Fig. 3B). During the 2 months of follow up, the patient's visual acuity decreased progressively, with the BCVA declining to 20/50 in OD and 20/60 in OS. SD-OCT showed more extensive disruption of the ellipsoid zone and the external limiting membrane in OU (Fig. 1C). There was no definite progression of mfERG abnormalities in OS, but a progression of delayed latency in OD with reduced amplitude in the inner segment ring was noted.
Based on the diagnosis of AIR, treatment was started with 1.0 g of high dose intravenous steroid pulse therapy for 3 days. Afterwards, the patient was placed on oral steroid and immunosuppressive agents for 5 weeks including 60 mg prednisolone, 200 mg cyclosporine A, and 100 mg azathioprine. Despite the immunosuppressive treatment, the patient's symptoms continued to worsen progressively with the BCVA decreasing to 20/120 in OD and 20/200 in OS from 20/40 in OU over a period of 4 months. There was a progression of central visual field loss on a 30-2 HVF exam (Fig. 1E). Posterior subtenon triamcinolone injection was performed in OU; however, the patient's condition failed to improve, and his visual acuity and visual field continued to deteriorate. The patient was subsequently lost to follow up, about 17 weeks after his first visit.
AIR is an autoantibody-induced retinopathy presumably caused by circulating autoantibodies targeted against retinal and retinal-like antigens. AIR can be classified as paraneoplastic AIR or nonparaneoplastic AIR. Paraneoplastic AIR results from the remote effects of diverse tumors and it includes CAR, MAR, and BDUMP. CAR revealed diffuse degeneration of photoreceptors in the outer retina,5 while MAR demonstrateed selective bipolar cell dysfunction in the inner retina,6 and BDUMP was characterized by diffuse uveal thickening of melanocytic proliferation.7 Nonparaneoplastic AIR, the most common form of AIR, can share similar clinical and electrophysiological characteristics with CAR1 but is not associated with an underlying malignancy. AIR patients present with acute or subacute bilateral visual loss. Because of its heterogeneous clinical features and near-normal appearance upon fundus examination and SD-OCT, AIR might escape diagnosis if it is not examined with high clinical suspicion. Full-field ERG could be normal in early-stage AIR; however, mfERG would detect the focal area of decreased amplitude.1 Various laboratory techniques, including immunohistochemistry, Western blot, and enzyme-linked immunosorbent assay, have been used to detect antiretinal antibodies which facilitate immune-mediated retinopathy in AIR. Anti-recoverin,8 α-enolase,9 carbonic anhydrase II,10 and rod transducin-α4 have been detected in nonparaneoplastic AIR.
AIR was suspected based on the clinical features of this case, including progressive visual loss over one year with normal fundus, color vision loss, central visual field defects, loss of the ellipsoid zone on SD-OCT, and mfERG abnormalities. As there was no definite evidence of tumor activity, the patient was diagnosed with presumed nonparaneoplastic AIR rather than CAR. This was supported by the detection of autoantibodies targeting various retinal antigens in serum samples. Retinopathies with hereditary, toxic, and inflammatory causes need to be differentiated, as they may share common clinical features.2 In this case, complete medical and oncologic examination including RP1L1 analysis was performed to rule out differential diagnosis of possible hereditary and other systemic causes.
There is presently no definitive treatment of choice for AIR, and the evidence for therapeutic intervention is limited.1 Long-term immunosuppression with systemic and local steroids have shown favorable outcomes in some cases of AIR.611 Other immunosuppressive agents8 and intravenous immunoglobulin6 treatment have also had limited success. In our case, both local and systemic immunosuppression was administered but did not lead to visual improvement. Once the destruction of the retinal photoreceptors progressed, immunosuppressive interventions produced very little visual improvement.
In conclusion, our report presents the first case of nonparaneoplastic AIR in Korea. Its clinical diagnosis was supported by positive Western blots for anti-retinal antibodies and by immunohistochemical staining of human retinal bipolar and ganglion cells. The early detection of anti-retinal autoantibodies followed by prompt and aggressive treatment with immunosuppressive agents may serve a significant role in identifying and treating those who are at risk of retinal deterioration and blindness from AIR.
Figures and Tables
Fig. 1
Fundus photographs, SD-OCT and visual field examination. (A) Color fundus photography of both eyes showing no apparent abnormalities. (B) Initial SD-OCT revealing blurring of ellipsoid zone at subfoveal region, more severe in the left than in the right eye. (C) Two months later, follow up SD-OCT demonstrating more progressed disruption of the ellipsoid zone across the entire fovea in both eyes. (D) Initial 30-2 HVF revealing central scotoma only in the left eye. (E) Follow up 30-2 HVF after 4 months displaying more profound cecocentral field deterioration in both eyes. SD-OCT, spectral domain-optical coherence tomography; HVF, humphrey visual field.
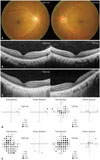
Fig. 2
Results of initial examinations: full-field electroretinography (A), and multifocal electroretinography (B) or (A) Initial full field ERG of the right eye displaying relatively intact responses, with slightly attenuated photopic a- and b-waves in the left eye. (B) Initial mfERG of the right eye revealing slightly reduced amplitude of inner segment ring and diminished amplitudes and delayed latencies of all modalities in the left eye. ERG, electroretinography; mfERG, multifocal ERG.

Fig. 3
Western blot and immunohistochemical staining results of patient's serum. (A) The patient's serum was positive for 23-kDa protein, enolase (46-kDa), aldolase (40-kDa), 62-kDa and 78-kDa proteins in Western blotting. The arrows indicate positive controls (C1: a positive control for recoverin; C2: a positive control for enolase). (B) Moderate immunohistochemical staining of some human retinal bipolar and ganglion cells as well as photoreceptor cells was noted. Scale bar=50 µm.
References
1. Grewal DS, Fishman GA, Jampol LM. Autoimmune retinopathy and antiretinal antibodies: a review. Retina. 2014; 34:827–845.
2. Heckenlively JR, Ferreyra HA. Autoimmune retinopathy: a review and summary. Semin Immunopathol. 2008; 30:127–134.

3. Weleber RG, Watzke RC, Shults WT, Trzupek KM, Heckenlively JR, Egan RA, et al. Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with antienolase antibodies. Am J Ophthalmol. 2005; 139:780–794.

4. Adamus G. Autoantibody targets and their cancer relationship in the pathogenicity of paraneoplastic retinopathy. Autoimmun Rev. 2009; 8:410–414.

5. Goetgebuer G, Kestelyn-Stevens AM, De Laey JJ, Kestelyn P, Leroy BP. Cancer-associated retinopathy (CAR) with electronegative ERG: a case report. Doc Ophthalmol. 2008; 116:49–55.

6. Keltner JL, Thirkill CE, Yip PT. Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: eleven new cases and a review of 51 previously published cases. J Neuroophthalmol. 2001; 21:173–187.

7. Gass JD, Gieser RG, Wilkinson CP, Beahm DE, Pautler SE. Bilateral diffuse uveal melanocytic proliferation in patients with occult carcinoma. 1990. Retina. 2003; 23:6 Suppl. 527–533.
8. Whitcup SM, Vistica BP, Milam AH, Nussenblatt RB, Gery I. Recoverin-associated retinopathy: a clinically and immunologically distinctive disease. Am J Ophthalmol. 1998; 126:230–237.

9. Adamus G, Ren G, Weleber RG. Autoantibodies against retinal proteins in paraneoplastic and autoimmune retinopathy. BMC Ophthalmol. 2004; 4:5.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download