Abstract
Purpose
Bronchiectasis is the main cause of hemoptysis. When patients with bronchiectasis develop hemoptysis, clinicians often perform bronchoscopy and bronchial washing to obtain samples for microbiological and cytological examinations. Bronchial washing fluids were analyzed from patients with bronchiectasis who developed hemoptysis, and the clinical impacts of these analyses were examined.
Materials and Methods
A retrospective observational study of patients who underwent fiberoptic bronchoscopy for hemoptysis in Seoul National University Bundang Hospital, a university affiliated tertiary referral hospital, between January 2006 and December 2010 were reviewed. Among them, patients who had bronchiectasis confirmed by computed tomography and had no definite cause of hemoptysis other than bronchiectasis were reviewed. The demographic characteristics, bronchoscopy findings, microbiological data, pathology results and clinical courses of these patients were retrospectively reviewed.
Results
A total of 130 patients were reviewed. Bacteria, non-tuberculous mycobacteria (NTM), and Mycobacterium tuberculosis were isolated from bronchial washing fluids of 29.5%, 21.3%, and 0.8% patients, respectively. Suspected causal bacteria were isolated only from bronchial washing fluid in 19 patients, but this analysis led to antibiotics change in only one patient. Of the 27 patients in whom NTM were isolated from bronchial washing fluid, none of these patients took anti-NTM medication during the median follow-up period of 505 days. Malignant cells were not identified in none of the patients.
Bronchiectasis is primarily the result of airway injury and remodeling caused by recurrent or chronic inflammation. Bronchiectasis can cause repeated respiratory infections, a disabling productive cough, shortness of breath, and occasional hemoptysis.1 Of these clinical manifestations, hemoptysis can be an alarming and life-threatening complication. Bronchiectasis is one of the major cause of hemoptysis.2 Fortunately, most patients with bronchiectasis do not develop hemoptysis beyond blood-streaked sputum. However, when persistent hemoptysis occurs, patients should receive antibiotics with careful work-up. When conservative measures or treatments fail, identification of the regional or specific source of bleeding may guide further therapy.3
The use of bronchoscopy to identify the sites and causes of bleeding in hemoptysis is well established. Bronchoscopy can localize the bleeding lobe in over 60% of cases and may be particularly helpful in cases in which alternative causes of bleeding are likely.4 Computed tomography (CT) and the analysis of symptoms can also help to localize the site of bleeding; however, bronchoscopy is a more definitive diagnostic test, especially when the lesion is diffuse.5 Therefore, many experts advocate the use of fiberoptic bronchoscopy as the primary method for localizing the site of bleeding in massive hemoptysis,6,7 although the timing and necessity of bronchoscopy before bronchial artery embolization is controversial.8
Inflammation is the main cause of hemoptysis irrespective of underlying diseases.9 Bronchiectasis is primarily a disease of the bronchi and bronchieoles involving a vicious circle of transmural infection and inflammation,10 and hemoptysis may result from erosive airway damage caused by an acute infection in patients with bronchiectasis.11 For these reasons, bronchial washing is sometimes performed to obtain samples for microbiological and cytological examinations during bronchoscopy in patients with bronchiectasis that have developed hemoptysis. However, the diagnostic yield and utility of this procedure have not been evaluated in detail. In this study, we analyzed bronchial washing fluids obtained from patients with bronchiectasis that had developed hemoptysis.
We reviewed patients retrospectively who underwent fiberoptic bronchoscopy for hemoptysis in Seoul National University Bundang Hospital, a university affiliated tertiary referral hospital, between January 2006 and December 2010. These patients received a bronchoscopy to identify the sites and evaluate etiology of hemoptysis. Of these, patients who had bronchiectasis confirmed by CT and had no definite cause of bleeding other than bronchiectasis, such as a tumor, were enrolled. CT data were reviewed by two pulmonologists (Drs. JHP and SWL) who reached a consensus about which patients to enroll. The demographic characteristics, bronchoscopy findings, microbiological data, pathology results, and clinical courses of these patients were retrospectively reviewed. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B-1106/129-116).
Flexible bronchoscopy and diagnostic techniques were performed under monitored anesthesia care as recommended by the American Thoracic Society and the British Thoracic Society.12,13 All bronchoscopic examinations were performed using a 4.9-5.9 mm flexible bronchoscope (BF-1T60t, Olympus, Tokyo, Japan) by experienced bronchoscopists (Drs. LSW, YHI, LJH and LCT) who described gross findings such as the presence of bleeding, tumor infiltration, or vascularity. Bronchial washing was performed at the site of the bronchiectatic lesion. After a full inspection of all the visible segmental and subsegmental bronchi, the flexible bronchoscope was wedged into a segmental bronchus leading to the bronchiectasis. Then, 10 mL of normal saline was repeatedly introduced until 20 mL of the aspirate was collected in the trap bottle.13,14,15
Cytological specimens and cultures were performed with standard method.16,17 Bronchial washing fluid specimens were immediately transported to the laboratory, decontaminated with 4% sodium hydroxide, homogenized, and concentrated by centrifugation (3000×g, 20 min). For cytological examination, direct smears were made from the sediment, which were subjected to hematoxylin and eosin staining and Papanicolaou staining. The cytological specimens were classified as benign, atypical, suspicious, or positive with respect to malignancy. The processed sediment was stained using the Ziehl-Neelsen method for acid-fasting bacilli. Mycobacterial cultures were performed in 3% Ogawa media at Seoul National University Bundang Hospital. We examined the cultures every week, and the cultures were recorded as negative if colonies were absent after 8 weeks of incubation.16 The Mycobacterium tuberculosis (MTB) complex was identified using a commercial DNA probe (Accu-Probe Mycobacterium Complex Culture Identification kit; Gen-Probe; San Diego, CA, USA). Nested PCR for MTB was performed using the ABSOLUTE™ MTB PCR kit (BioSewoom Inc., Seoul, Korea) according to the manufacturer's instructions. Respiratory samples were gram-stained and homogenized. Undiluted and serial-diluted secretions were plated on blood, MacConkey Agar, and selective media (containing vancomycin, bacitracin, and clindamycin). The growth of cultures was evaluated after 24 h and 48 h. Microorganisms were identified using standard methods.17,18 All examination for bronchial washing fluid was performed by pathologists and technicians with experience in this field for more than five years.
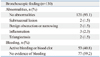
A total of 292 patients that presented with hemoptysis underwent fiberoptic bronchoscopy during the study period. Of these, 130 patients who had bronchiectasis confirmed by CT and had no definite cause of bleeding other than bronchiectasis were included in the study. Fifty two (40%) patients were male and the median age was 56 years (range; 30-83 years). Eighty six patients (66.2%) were nonsmokers, 32 patients (24.6%) were ex-smokers, and 12 patients were non-smokers. Forty-seven (36.2%) patients had a previous history of pulmonary tuberculosis (TB) and four patients had an underlying disease associated with bronchiectasis. Three patients had pertussis and one patient had measles. Fifty two (40%) patients showed involvement of bronchiectasis in more than one lobe. The median follow-up period was 303 days (range: 0-1185 days) (Table 1).
Bronchoscopy results detected no abnormalities in 121 (93.1%) patients. Two (1.5%) patients had submucosal lesions including small nodular lesions; two (1.5%) patients had benign obstructions or narrowing, three (2.3%) patients had inflammation including edematous and hyperemic mucosal lesions, and two (1.5%) patients had telangiectasia. The site of bleeding was confirmed by the detection of active bleeding or a blood clot in 53 (40.8%) patients, whereas there was no evidence of bleeding in 77 (59.2%) patients (Table 2).
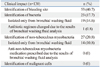
Bronchial washing of the bronchiectatic lesion was performed for each of the 130 patients. Bacterial culture of the bronchial washing fluids was performed on 78 patients, and acid-fast bacilli smears and mycobacterial cultures of the bronchial washing fluids were performed for 127 patients. Sixty four patients (49.2%) received antibiotics before bronchial washing. The median antibiotic use was 3 days (range: 1-12 days). The proportion of isolated bacteria was not different among patients whether they received pre-bronchoscopic antibiotics or not. Bacteria were isolated in 12 (28.6%) patients who received antibiotics before bronchoscopy. In patients who did not receive antibiotics, bacteria were isolated in 11 (29.7%) patients. Bacteria were isolated from the bronchial washing fluids of 23 (29.5%) patients as follows; Pseudomonas aeruginosa (10 patients, 12.8%), Staphylococcus aureus (eight patients, 10.2%), Klebsiella pneumoniae (two patients, 2.6%), Enterobacter aerogenes (two patients, 2.6%), and Ochrobactrum anthropi (one patient, 1.3%). Non-tuberculous mycobacteria (NTM) were isolated from the bronchial washing fluids of 27 (21.3%) patients, including Mycobacterium abscessus (11 patients, 8.7%), Mypacobacterium avium (five patients, 3.9%), and Mycobacterium intracellulare (five patients, 3.9%). MTB was isolated from the bronchial washing fluid of one (0.8%) patient (Table 3). This patient had been diagnosed with multidrug-resistant (MDR) TB before hemoptysis developed, and a nodular lesion indicative of TB was detected by CT. PCR of MTB was performed using the bronchial washing fluids of 101 patients, and the results of two patients were positive. The one was the MDR TB patient, as mentioned above. The other patient did not take anti-TB medication because TB was not suspected clinically and radiologically. He did not exhibit clinical or radiological deterioration during the follow-up period of 550 days.
Of the 27 patients in whom NTM were isolated from bronchial washing fluids, NTM were not isolated from the sputum of 14 (51.9%) patients (Table 4). Diagnostic concordance of sputum and bronchial washing fluid was moderate agreement; kappa ratio 0.423 (p<0.001). None of these 14 patients took anti-NTM medication during the median follow-up period of 505 days (range: 0-970 days). Board certified pulmonologists (Drs. CTL, JHL, HIY, and SWL) followed-up these patients and decided not to prescribe anti-NTM medication as they judged that there was no evidence of bronchiectasis progression or consolidation by chest radiography.19 NTM were isolated from both the sputum and bronchial washing fluids of the other 13 patients. Of these, six patients took anti-NTM medication because they judged NTM lung disease by board certified pulmonologists, whereas the other seven patients did not take anti-NTM medication because there was no evidence of disease progression by chest radiography.
Of the 23 patients in whom suspected causal bacteria were isolated from bronchial washing fluids, bacteria were not isolated from the sputum of 19 (82.6%) patients (Table 5). Diagnostic concordance of sputum and bronchial washing fluid was slight agreement; kappa ratio 0.058 (p=0.499). 13 patients received antibiotics due to suspect pneumonia. The antibiotic regimen of one of these 19 patients who only isolated bacteria in bronchial washing fluid was changed from cefpodoxime to ciprofloxacin because Enterobacter aerogenes resistant to cefazolin and cefoxitin was identified in their bronchial washing fluid. Of the other 18 patients, eight had taken antibiotics to treat combined pneumonia. The initial treatment regimens of these patients included third generation cephalosporin (five patients), third generation cephalosporin plus macrolide (one patient), macrolide (one patient), and β-lactam plus a β-lactamase inhibitor (one patient). None of the bacteria identified in the bronchial washing fluids of these eight patients were resistant to the antibiotics currently being prescribed, and so the antibiotic regimens of these patients were not changed. Cytological examinations of the bronchial washing fluids did not detect malignant cells in any patients (Table 6).
Fiberoptic bronchoscopy is not routinely performed in patients with bronchiectasis; however, it is sometimes used to identify bacteria or mycobacteria, particularly when the patient also has chronic respiratory symptoms such as coughing and mucopurulent sputum.11 Bronchoscopy should also be considered in patients with significant hemoptysis.20 Although the optimal time at which bronchoscopy should be performed remains controversial, this is the primary method for the diagnosis and localization of hemoptysis in patients with bronchiectasis.21
Many pulmonologists analyze bronchial washing fluids to evaluate the etiology of hemoptysis by microbiological and cytological examinations.20,21 However, the use of bronchial washing to analyze hemoptysis in patients with bronchiectasis is not well established. Many patients with bronchiectasis develop hemoptysis due to repeated infections and inflammation. Analysis of bronchial washing fluids of patients with bronchiectasis and bleeding may provide important information about the etiology of hemoptysis. However, our study raises doubts about the clinical usefulness of bronchial washing in this context.
In the current study, bacteria were isolated from the bronchial washing fluids of 29.5% patients; however, bacteria were not isolated from the sputum in 82.6% of these patients. The most common bacteria detected were P. aeruginosa and S. aureus, similar to previous reports.11 However, in the majority of cases, hemoptysis and lung infiltration had already been improved by treatment with empirical antibiotics based on third generation cephalosporin when these bacteria were isolated, and the result of bronchial washing fluids did not affect the antibiotic regimens except one case. The identification of microorganisms in bronchial washing fluids may assist the treatment of future complications such as hemoptysis and pneumonia, but pathogens can be also isolated by analysis of sputum samples alone in 77-88% of patients with bronchiectasis.20,22 Antibioitcs are recommended when patients with bronchiectasis have purulant sputum.23 Meanwhile, when hemoptysi is the main presentation, the additional role of bronchial washing fluid to sputum exam could be low due to relatively small amount of bacterial load. In our study, about 50% of patients received antibiotics before bronchoscopy, which could also affect low bacterial isolation rate from bronchial washing.
The most common NTM isolated in this study were M. abscessus and the Mycobacterium avium complex (MAC), which comprises M. avium and M. intracellulare. The prevalence of different NTM species exhibits marked geographic variability.24,25 The MAC (32%) and M. abscessus (29%) are the most common NTM in Korea.26 Of the 27 (20.8%) patients in whom NTM were isolated from bronchial washing fluids, NTM were not detected in the sputum of 14 (51.9%) patients. None of these 14 patients took anti-NTM medication during the median follow-up period of 505 days because they did not exhibit any clinical deterioration. This suggests that analysis of bronchial washing fluid sample in a single event of hemoptysis cannot determine the clinical significance.19 NTM is a chronic inflammatory disease and we should take into account the potential risks and benefits before anti-NTM treatment, which necessitates chronic respiratory symptom such as cough and sputum with radiographic aggravation.27
Cytological examinations of bronchial washing fluids of each of the enrolled patients were performed; however, no malignant cells were identified. Although hemoptysis can be a symptom of malignancy, the possibility that bronchial washing can detect a hidden malignancy that is not detected by CT scanning seems unlikely.
Flexible bronchoscopy is a safe procedure as long as basic precautions are taken.28 Bronchial lavage to obtain microbiological specimens appears to be a relatively safe procedure that does not cause lasting or serious sequelae.29 However, bronchoalveolar lavage can cause marked hypoxemia, particularly when the volume of lavage fluid is large.30 Post-bronchoscopic fever is more common after bronchoalveolar lavage (10-30% patients)13,31 than after routine bronchial lavage (1.2% patients).32 Analysis of bronchial washing fluids including cytology, culture, and molecular genetics can be expensive. To prevent futile procedures being performed, clinicians should consider these additional costs and balance them against the potential benefits to the patient.
This study has several limitations. First, not all patients with bronchiectasis and hemoptysis at the hospital were included; only patients who underwent bronchoscopy were reviewed. Patients with bronchiectasis and hemoptysis that did not undergo a bronchoscopy were excluded. However, the extent of hemoptysis in these patients is unlikely to have been clinically significant. Second, the follow-up duration might have been insufficient to evaluate the clinical significance of the identified NTM fully. A longer follow-up duration may have resulted in the antibiotic regimens of some patients being changed. However, the mean follow-up period in this study was 18.5 months which is longer than the follow-up period of a similar study.33 Third, this is the retrospective observational study and a prospective study including large number of patients can be necessary to guide clinical practice. For this limitation, we could not get enough information about the volume of hemoptysis, which was one of important clinical parameter in hemoptysis. However, our study still has meaning because there is no large randomized study which can conclude this issue until now, and it can also be the basis of a future study.
In conclusion, bronchial washing can be a useful method to identify microorganisms in patients with bronchiectasis that develop hemoptysis. However, these results do not markedly affect the clinical decisions taken.
Figures and Tables
Table 4
Comparison of the Number of Sputum and Bronchial Washing Fluid Samples in Which Non-Tuberculous Mycobacteria Were Isolated by Acid-Fast Bacilli Smears

ACKNOWLEDGEMENTS
This study was supported by a grant of the Korean Health Technology R&D project, Ministry for Health, Welfare & Family Affairs, and Republic of Korea (A120301).
References
1. O'Donnell AE. Bronchiectasis. Chest. 2008; 134:815–823.
2. Soares Pires F, Teixeira N, Coelho F, Damas C. Hemoptysis--etiology, evaluation and treatment in a university hospital. Rev Port Pneumol. 2011; 17:7–14.
4. Swanson KL, Johnson CM, Prakash UB, McKusick MA, Andrews JC, Stanson AW. Bronchial artery embolization : experience with 54 patients. Chest. 2002; 121:789–795.
5. Hsiao EI, Kirsch CM, Kagawa FT, Wehner JH, Jensen WA, Baxter RB. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol. 2001; 177:861–867.

6. Dweik RA, Stoller JK. Role of bronchoscopy in massive hemoptysis. Clin Chest Med. 1999; 20:89–105.

7. Cahill BC, Ingbar DH. Massive hemoptysis. Assessment and management. Clin Chest Med. 1994; 15:147–167.
8. Ramakantan R, Bandekar VG, Gandhi MS, Aulakh BG, Deshmukh HL. Massive hemoptysis due to pulmonary tuberculosis: control with bronchial artery embolization. Radiology. 1996; 200:691–694.

9. Johnson JL. Manifestations of hemoptysis. How to manage minor, moderate, and massive bleeding. Postgrad Med. 2002; 112:101–106.
10. Cole PJ. Inflammation: a two-edged sword--the model of bronchiectasis. Eur J Respir Dis Suppl. 1986; 147:6–15.
12. Goldstein RA, Rohatgi PK, Bergofsky EH, Block ER, Daniele RP, Dantzker DR, et al. Clinical role of bronchoalveolar lavage in adults with pulmonary disease. Am Rev Respir Dis. 1990; 142:481–486.

13. British Thoracic Society Bronchoscopy Guidelines Committee, a Subcommittee of Standards of Care Committee of British Thoracic Society. British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax. 2001; 56:Suppl 1. i1–i21.
14. Pasteur MC, Bilton D, Hill AT. British Thoracic Society Non-CF Bronchiectasis Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010; 65:577.

15. Lee HS, Kwon SY, Kim DK, Yoon HI, Lee SM, Lee JH, et al. Bronchial washing yield before and after forceps biopsy in patients with endoscopically visible lung cancers. Respirology. 2007; 12:277–282.

16. Kumari S, Ichhpujani RL. World Health Organization. Regional Office for South-East Asia. Guidelines on standard operating procedures for Microbiology. New Delhi: SEARO WHO;2000.
17. Standards ATSCoRoD. Diagnostic standards and classification of tuberculosis in adults and children. Atlanta: US Department of Health and Human Services, Public Health Service;2000.
18. Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA. Manual of Clinical Microbiology. 9th ed. Washington DC: American Society of Microbiology;2007.
19. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007; 175:367–416.

20. Nicotra MB, Rivera M, Dale AM, Shepherd R, Carter R. Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest. 1995; 108:955–961.

21. Chun JY, Morgan R, Belli AM. Radiological management of hemoptysis: a comprehensive review of diagnostic imaging and bronchial arterial embolization. Cardiovasc Intervent Radiol. 2010; 33:240–250.

22. Pasteur MC, Helliwell SM, Houghton SJ, Webb SC, Foweraker JE, Coulden RA, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med. 2000; 162(4 Pt 1):1277–1284.

23. Hill AT, Pasteur M, Cornford C, Welham S, Bilton D. Primary care summary of the British Thoracic Society Guideline on the management of non-cystic fibrosis bronchiectasis. Prim Care Respir J. 2011; 20:135–140.

24. Simons S, van Ingen J, Hsueh PR, Van Hung N, Dekhuijzen PN, Boeree MJ, et al. Nontuberculous mycobacteria in respiratory tract infections, eastern Asia. Emerg Infect Dis. 2011; 17:343–349.

25. Marras TK, Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med. 2002; 23:553–567.
26. Koh WJ, Kwon OJ, Jeon K, Kim TS, Lee KS, Park YK, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006; 129:341–348.

27. Daley CL, Griffith DE. Pulmonary non-tuberculous mycobacterial infections. Int J Tuberc Lung Dis. 2010; 14:665–671.
28. Zavala DC. Diagnostic fiberoptic bronchoscopy: techniques and results of biopsy in 600 patients. Chest. 1975; 68:12–19.
29. Hertz MI, Woodward ME, Gross CR, Swart M, Marcy TW, Bitterman PB. Safety of bronchoalveolar lavage in the critically ill, mechanically ventilated patient. Crit Care Med. 1991; 19:1526–1532.

30. Pirozyński M, Sliwiński P, Radwan L, Zieliński J. Bronchoalveolar lavage: comparison of three commonly used procedures. Respiration. 1991; 58:72–76.

31. de Fijter JW, van der Hoeven JG, Eggelmeijer F, Meinders AE. Sepsis syndrome and death after bronchoalveolar lavage. Chest. 1993; 104:1296–1297.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download