Abstract
Objective
To evaluate the diagnostic outcomes of ultrasonography-guided core needle biopsy (US-CNB), US-guided vacuum-assisted biopsy (US-VAB), and stereotactic-guided vacuum-assisted biopsy (S-VAB) for diagnosing suspicious breast microcalcification.
Materials and Methods
We retrospectively reviewed 336 cases of suspicious breast microcalcification in patients who subsequently underwent image-guided biopsy. US-CNB was performed for US-visible microcalcifications associated with a mass (n = 28), US-VAB for US-visible microcalcifications without an associated mass (n = 59), and S-VAB for mammogram-only visible lesions (n = 249). Mammographic findings, biopsy failure rate, false-negative rate, and underestimation rate were analyzed. Histological diagnoses and the Breast Imaging Reporting and Data System (BI-RADS) categories were reported.
Results
Biopsy failure rates for US-CNB, US-VAB, and S-VAB were 7.1% (2/28), 0% (0/59), and 2.8% (7/249), respectively. Three false-negative cases were detected for US-CNB and two for S-VAB. The rates of biopsy-diagnosed ductal carcinoma in situ that were upgraded to invasive cancer at surgery were 41.7% (5/12), 12.9% (4/31), and 8.6% (3/35) for US-CNB, US-VAB, and S-VAB, respectively. Sonographically visible lesions were more likely to be malignant (66.2% [51/77] vs. 23.2% [46/198]; p < 0.001) or of higher BI-RADS category (61.0% [47/77] vs. 22.2% [44/198]; p < 0.001) than sonographically invisible lesions.
Mammographically visible suspicious microcalcifications with no associated mass are usually diagnosed by stereotactic or excisional biopsy under wire localization (123). Ultrasonography (US) is limited for detecting microcalcifications, which is related to low contrast resolution; however, US detects microcalcifications associated with other findings, such as mass or ductal changes (456). In general, patients prefer sonographically-guided procedures to mammographically-guided procedures, as patients tend to be more comfortable in the supine position, the breast is not compressed, and the procedure is less time-consuming. In addition, no ionizing radiation is used, the needle insertion site is more flexible, and real-time observations can be made (78). For these reasons, if microcalcifications are detected on US, US-guided core biopsy (US-CNB) or US-guided vacuum-assisted biopsy (US-VAB) is conducted for the diagnosis (591011).
When all of these findings are considered, the histopathological diagnosis is determined for suspicious microcalcifications detected on mammography using a variety of biopsy methods, including stereotactic VAB (S-VAB), US-CNB, US-VAB, or surgical biopsy, depending on the imaging findings and each patient's clinical situation.
Several studies have reported on the diagnostic outcomes of stereotactic biopsy (121314) and US-guided biopsy (591015). However, no study has considered the overall consequences of image-guided methods for diagnosing microcalcifications.
The purpose of this study was to evaluate the success rate, malignancy rate, and diagnostic accuracy of US-CNB, US-VAB, and S-VAB for diagnosing suspicious microcalcifications detected on mammography.
This single-center retrospective study was approved by the Institutional Review Board of our hospital, and signed informed consent was obtained from all patients prior to their biopsy procedures.
A retrospective review was performed of 10310 patients who underwent mammography between March 2009 and January 2011 at our institution. A total of 1836 patients were classified under Breast Imaging Reporting and Data System (BI-RADS) categories 4 and 5, and among these, 406 patients with 407 lesions had microcalcifications without associated findings. All of these cases were recommended for biopsy; 15 patients were lost during follow-up or decided not to undergo biopsy. Fifty-five patients with 56 lesions underwent surgical biopsies because of other scheduled surgeries or because of patient preference. Finally, 336 patients with 336 lesions who underwent image-guided biopsy were included in our study. Of the 336 patients, 28 underwent US-CNB, 59 underwent US-VAB, and 249 underwent S-VAB (Fig. 1).
Mammography was performed with the GE Senographe DS full-field digital mammography system (GE Healthcare, Milwaukee, WI, USA) and the Lorad/Hologic Selenia full-field digital mammography system (Lorad/Hologic, Danbury, CT, USA). Standard craniocaudal and mediolateral oblique views were routinely obtained along with magnified mammograms.
Ultrasonography was performed using a high-resolution US unit (iU22, Philips Medical Systems, Bothell, WA, USA or Logiq 9, GE Medical Systems, Milwaukee, WI, USA) with 12-MHz linear-array transducers by one of nine dedicated radiologists with 1-18 years of experience. All suspicious microcalcification clusters were evaluated on sonography before biopsy.
The management protocol for suspicious microcalcifications without other mammographic features in our institution is US-CNB for microcalcifications with an associated mass (Fig. 2) and US-VAB for microcalcifications visible on US without an associated mass. If the correlation between US-visible microcalcifications and mammographically observed microcalcifications is uncertain, a radio-opaque marker is placed on the skin at the location of the US-visible microcalcification. The area is then reevaluated to confirm if the US-visible microcalcifications can be visualized on mammography (Fig. 3). S-VAB is used for microcalcifications visible only on mammography. The number of core samples is decided by each operator. An excisional biopsy was performed if the patient preferred surgical excision or was scheduled for another surgery. Except in cases where a patient or clinician requested a different procedure, all lesions included in this study were managed according to the above protocol.
All biopsy procedures were performed by one of nine dedicated radiologists with 1-18 years of experience. US-CNB was performed using a 14-gauge core needle (Stericut; TSK Laboratory, Tochigi, Japan). US-VAB and S-VAB were performed using 8- or 11-gauge vacuum probes (Mammotome; Ethicon Endo-Surgery, Inc., Cincinnati, OH, USA). S-VAB was performed in the lateral decubitus position (16) with the breast lesion facing up (Digital Stereotaxy with Senographe DS Interventional; GE Healthcare). A microclip was inserted at the biopsy site after S-VAB only. Specimen mammography was performed after biopsy in every case to confirm whether the microcalcifications of interest were successfully retrieved. All specimens with and without calcifications were sent separately for pathological examination.
In all cases, the histopathological results were correlated with mammographic findings by radiologists and pathologists. In cases of discordance, repeat biopsy or surgical excision was recommended. Patients with high-risk lesions, such as atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), lobular carcinoma in situ (LCIS), papilloma, radial scar, or malignant lesions, were recommended for surgical resection. Patients with concordant benign lesions were scheduled for repeat mammography at 6, 12, and 24 months.
The clinical, histological, and imaging findings were reviewed, including the results from each biopsy and follow-up imaging studies. The size, number, distribution, morphology, and final BI-RADS category of the microcalcifications on mammography were compared among the three biopsy method groups. Biopsy failure was defined as technical failure of targeting or sampling of microcalcifications because of a vasovagal reaction in a patient, inability to visualize or access the target during biopsy, or absence of calcification on specimen radiography.
We evaluated the malignancy rate, histological underestimation (17), and false-negative results, which were based on patients who underwent surgery after biopsy or who were followed-up with mammography for at least 1 year after being diagnosed with a benign lesion. The malignancy rate was determined by dividing the number of biopsy-diagnosed ductal carcinoma in situ (DCIS) cases and invasive carcinoma cases by the total number of successfully performed biopsies that were followed by surgery or by more than 1 year of mammographic follow-up. The ADH underestimation rate was defined as the number of biopsy-diagnosed ADH lesions that were upgraded to DCIS or invasive cancer at surgical excision divided by the number of biopsy-diagnosed ADH lesions. The DCIS underestimation rate was defined as the number of DCIS lesions upgraded to invasive cancer at surgical excision divided by the number of biopsy-diagnosed DCIS lesions. A false-negative result was defined as a final diagnosis of malignancy for a lesion previously diagnosed as benign by biopsy. The false-negative rate was also defined as the number of false-negative cases divided by the number of cases with a final diagnosis of malignancy. We also compared US-visible and US-invisible lesions by malignancy rate and BI-RADS category.
The chi-square test was performed to correlate the BI-RADS final assessment category and malignancy rate with visibility on US and to compare the proportion of invasive carcinomas showing malignant pathology according to biopsy method. Statistical analyses were performed using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). P values < 0.05 were considered statistically significant.
The mean age of the 336 patients was 47.5 years (range, 23-68 years). The mammographic features are summarized in Table 1. The breast tissue of most patients showed a heterogeneously dense pattern. The assigned BI-RADS assessment category differed according to biopsy method. Category 4a was the most commonly assigned category in the US-VAB and S-VAB groups, whereas category 4c was the most commonly assigned in the US-CNB group. The mean number of specimens obtained per lesion was 6.6 (range, 4-15) with US-CNB; 12.0 (range, 6-33) with US-VAB; and 12.6 (range, 3-53) with S-VAB. The US features of the 28 US-CNB lesions are summarized in Table 2.
A total of 275 patients underwent surgery after biopsy or were followed-up for at least 1 year with mammography after being diagnosed with a benign lesion. Of these 275 lesions, 97 were malignant on pathology (21 cases of invasive cancer and 76 cases of DCIS), and 178 were benign. Of the benign lesions, benignity was confirmed by surgical excision in 17 cases and presumed in 161 cases by the stability observed on follow-up mammography, with a mean follow-up period of 1173.8 days (range, 385-1924 days).
Of the 28 US-CNB lesions, biopsy failed in two patients (7.1%) because the calcifications were absent on specimen radiography. These two patients underwent re-biopsy, one with S-VAB following benign pathology and one with excisional biopsy following a DCIS result. Seven procedures failed (2.8%) during S-VAB for the 249 lesions because of vasovagal reactions (n = 2) or inaccessibility of lesions due to inadequate breast thickness under compression (n = 5). These seven patients underwent excisional biopsies, and all results were benign. No US-VAB biopsy failures occurred.
Of the 327 successful biopsy cases, the histological results of the initial biopsy were benign in 217 cases; 214 were considered concordant with the imaging findings and three were considered discordant with benign results. Three patients underwent re-biopsy, two with US-VAB and one with excisional biopsy, and the final pathological results were all DCIS.
The malignancy rates according to BI-RADS category were 17.9% (33/184) for category 4a, 53.2% (25/47) for category 4b, 84.8% (28/33) for category 4c, and 100% (11/11) for category 5. The malignancy rates were 83.3%, 58.5%, and 23.7% for US-CNB, US-VAB, and S-VAB, respectively. Among lesions with malignant pathology, invasive carcinoma was more frequent in the US-CNB group (45.0%, 9/20) than in the US-VAB (16.1%, 5/31) and S-VAB groups (17.4%, 8/46).
The false-negative rates were 15.0% (3/20), 0% (0/31), and 4.3% (2/46) for US-CNB, US-VAB, and S-VAB, respectively. Three false-negative cases for US-CNB and two false-negative cases for S-VAB are summarized in Table 3. Four patients underwent immediate re-biopsy due to the absence of calcifications on specimen mammography (n = 1) (Fig. 4) and image-pathology discordance (n = 3), and all were DCIS. The other case was thought to be image-pathology concordant after benign biopsy results. The patient's screening mammography 3 years later revealed an increased extent of the previous suspicious calcifications, and the re-biopsy result was DCIS. The ADH underestimation rates were 0% (0/1) for US-CNB, 0% (0/2) for US-VAB, and 20% (1/5) for S-VAB. The DCIS underestimation rates were 41.7% (5/12), 12.9% (4/31), and 8.6% (3/35) for US-CNB, US-VAB, and S-VAB, respectively.
For the other high-risk lesions that were excised, one ALH diagnosis at S-VAB was confirmed to be ALH after excision. Three cases of LCIS at S-VAB were confirmed to be malignant (two DCIS and one mixed solid papillary carcinoma) after excision.
The correlation between visibility on US and the BI-RADS final assessment category or histological diagnosis is shown in Table 4. Malignant pathology and a higher BI-RADS category were more frequent in US-visible lesions (p < 0.001). However, with regard to the proportion of cases showing malignant pathology, invasive cancer was 25.5% (13/51) in US-visible lesions and 17.4% (8/46) in US-invisible lesions (p = 0.334).
Our results demonstrate the overall diagnostic outcomes of US-CNB, US-VAB, and S-VAB. No previous study has reported overall outcomes of image-guided breast biopsy methods after establishing clear criteria at a single center during the same timeframe, although there have been many reports summarizing biopsy methods individually or comparing two biopsy methods (2591012131415).
The microcalcification retrieval rates in our study were 92.9% for US-CNB and 100% for US-VAB and S-VAB, which were within the range of previous studies (5101115). Among the seven biopsy failure cases (2.8%) in S-VAB, biopsy was technically infeasible in five lesions (2.0%) due to inadequate breast thickness under compression, and two patients (0.8%) developed vasovagal reactions. Previous studies reported technical feasibility rates of 93-99% (181920) and vasovagal reaction rates of 0-2% in the prone position for S-VAB (1218). The biopsy failure rate for S-VAB can differ according to patient position. The prone position, which is used in most institutions, is reliable and accurate but requires significant space and is relatively expensive. Some institutions prefer the sitting or decubitus position, but they can present problems due to the relatively high frequency of vasovagal reactions and patient motion (161821). Digital breast tomosynthesis-guided biopsies are performed at some institutions using the lateral decubitus position, and a higher biopsy success rate in less procedure time was reported compared with prone S-VAB (20).
We had five false-negative cases; three using US-CNB and two for S-VAB; one case was a calcification retrieval failure case, three cases were detected by imaging-histologic discordance, and the other case was detected by follow-up mammography. Of these five cases, four patients underwent repeat biopsy within 2 weeks, and the fifth underwent repeat biopsy 3 years later, demonstrating that an immediate imaging-histological correlation and long-term follow-up are vital for confirming a benign diagnosis after percutaneous imaging-guided breast biopsy. Previous studies reported missed false-negative cases that were detected at follow-up mammography and obtained 6-24 months after stereotactic biopsy (2223) and 6-27 months after US-CNB (24). The standard follow-up period after percutaneous breast biopsy has not been decided. Lee et al. (23) suggested up to 36 months of follow-up mammography after a nonspecific benign diagnosis by stereotactic biopsy. The mean follow-up period in our study was 1173.8 days (range, 385-1924 days), which may have been insufficient for confirming benign pathology in some patients.
In the current study, the ADH underestimation rate was 12.5% after S-VAB, which concurs with the reported range of 7-35% after S-VAB (10). The DCIS underestimation rates of US-VAB (41.7%) and S-VAB (8.6%) in the current study are consistent with those of previous reports (1012152526). However, the DCIS underestimation rate of US-CNB (41.7%) was higher than that of a previous report (12.5%) (11). This can be explained by the performance of US-CNB in cases of microcalcifications associated with a mass in which there is a higher chance of an invasive component.
Sonographically visible lesions were significantly more likely to be malignant (66.2% vs. 23.2%; p < 0.001) and have a higher BI-RADS category (61.0% vs. 22.2%; p < 0.001) than those not detected on US in our study, which concurs with previous reports that demonstrated a correlation between malignant pathology and visibility on US (5927). The proportion of malignant pathology was different according to biopsy method in our study. Invasive carcinoma was more frequent on US-CNB (45.0%, 9/20) than for US-VAB (16.1%, 5/31) and S-VAB (17.4%, 8/46), which was due to selecting US-visible masses for US-CNB. Previous studies have suggested that a mass seen on US will likely have invasive components (527). Although the malignancy rate was higher for US-VAB than that for S-VAB (58.5% vs. 23.7%), the proportion of invasive carcinoma was similar for US-VAB and S-VAB (16.1% vs. 17.4%). This result suggests that the presence of US-visible calcifications implies a more malignant pathology but does not increase the likelihood of invasion in cases with no accompanying mass. Echogenic foci within a mass or a ductal change is seen in DCIS on US, accompanied by internal microlobulations or a branch pattern distribution (28), so it is not surprising that visibility of calcifications on US is not associated with cancerous invasion.
Our study had several limitations. First, the number of patients was small, and the study was performed in a single center. Further study with a larger number of patients from multiple centers is needed to compare the overall diagnostic outcomes of the various biopsy methods. Second, we set the standard follow-up period as at least 1 year for mammography, which may be insufficient for diagnosing benign microcalcifications. Lastly, breast US is an operator-dependent procedure and reproducibility is dependent on individual skill of the radiologist. Inter-operator variability was not evaluated in this study.
In conclusion, US-VAB was a more accurate and acceptable biopsy method than US-CNB when suspicious microcalcifications were visualized on US. Calcifications showing malignant pathology or lesions assigned to a higher BI-RADS category were significantly more visible on US than benign lesions or lesions assigned to a lower BI-RADS category.
Figures and Tables
Fig. 1
Study population.
BI-RADS = Breast Imaging Reporting and Data System, S-VAB = stereotactic-guided vacuum assisted biopsy, US-CNB = ultrasonography-guided core needle biopsy, US-VAB = ultrasonography-guided vacuum assisted biopsy

Fig. 2
68-year-old female patient with complaint of bloody nipple discharge from left breast.
A. Magnified mammogram of left breast reveals suspicious segmental coarse heterogeneous microcalcifications. B. Ultrasonogram of left breast shows hypoechoic mass with internal hyperechoic foci in upper outer aspect of left breast. 14-gauge ultrasonography-guided core needle biopsy was performed on mass, and final diagnosis was invasive ductal carcinoma.
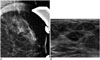
Fig. 3
54-year-old female patient who underwent screening mammography.
A. Magnified mammogram of left breast reveals suspicious segmental coarse heterogeneous microcalcifications. B. Ultrasonogram of left breast shows microcalcifications (arrows) at corresponding area of microcalcifications observed on mammography. C. Radio-opaque marker was placed on skin over lesion for confirmation, and left breast view was magnified. Ultrasonography (US)-visible microcalcifications are correlated with mammographically visualized microcalcifications. D. 11-gauge US-guided vacuum-assisted biopsy is performed. E. Specimen mammography confirms retrieval of microcalcifications. Diagnosis was ductal carcinoma in situ, which was also confirmed by post-partial mastectomy pathology.
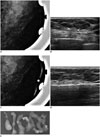
Fig. 4
47-year-old female patient who underwent screening mammography.
A. Magnified mammogram of left breast reveals suspicious grouped fine pleomorphic microcalcifications (arrow). B. Ultrasonogram of left breast shows hypoechoic mass with internal hyperechoic foci (arrows) in upper central aspect of left breast. 14-gauge ultrasonography-guided core needle biopsy was performed on mass, and calcifications were not retrieved per specimen mammography. Diagnosis was stromal fibrosis, which was thought to be discordant with imaging findings. Patient underwent excisional biopsy under wire localization, and final diagnosis was ductal carcinoma in situ.
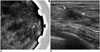
Table 1
Mammographic Features of Microcalcifications According to Biopsy Methods, n (%)

Table 2
US Features of US-CNB Lesions, n (%)

Table 3
False-Negative Diagnoses after Image-Guided Biopsy

Table 4
Correlation of US Visibility and Histologic Diagnosis of Mammographically Detected Microcalcifications
References
1. Anania G, Bazzocchi M, di Loreto C, Risaliti A, Terrosu G, Donini A, et al. Percutaneous large core needle biopsy versus surgical biopsy in the diagnosis of breast lesions. Int Surg. 1997; 82:52–55.
2. Elvecrog EL, Lechner MC, Nelson MT. Nonpalpable breast lesions: correlation of stereotaxic large-core needle biopsy and surgical biopsy results. Radiology. 1993; 188:453–455.
3. Gisvold JJ, Goellner JR, Grant CS, Donohue JH, Sykes MW, Karsell PR, et al. Breast biopsy: a comparative study of stereotaxically guided core and excisional techniques. AJR Am J Roentgenol. 1994; 162:815–820.
4. Park JS, Park YM, Kim EK, Kim SJ, Han SS, Lee SJ, et al. Sonographic findings of high-grade and non-high-grade ductal carcinoma in situ of the breast. J Ultrasound Med. 2010; 29:1687–1697.
5. Soo MS, Baker JA, Rosen EL. Sonographic detection and sonographically guided biopsy of breast microcalcifications. AJR Am J Roentgenol. 2003; 180:941–948.
6. Yu PC, Lee YW, Chou FF, Wu SC, Huang CC, Ng SH, et al. Clustered microcalcifications of intermediate concern detected on digital mammography: ultrasound assessment. Breast. 2011; 20:495–500.
7. Parker SH, Burbank F. A practical approach to minimally invasive breast biopsy. Radiology. 1996; 200:11–20.
8. Soo MS, Baker JA, Rosen EL, Vo TT. Sonographically guided biopsy of suspicious microcalcifications of the breast: a pilot study. AJR Am J Roentgenol. 2002; 178:1007–1015.
9. Cho N, Moon WK, Cha JH, Kim SM, Jang M, Chang JM, et al. Ultrasound-guided vacuum-assisted biopsy of microcalcifications detected at screening mammography. Acta Radiol. 2009; 50:602–609.
10. Kim HS, Kim MJ, Kim EK, Kwak JY, Son EJ, Oh KK. US-guided vacuum-assisted biopsy of microcalcifications in breast lesions and long-term follow-up results. Korean J Radiol. 2008; 9:503–509.
11. Yi J, Lee EH, Kwak JJ, Cha JG, Jung SH. Retrieval rate and accuracy of ultrasound-guided 14-G semi-automated core needle biopsy of breast microcalcifications. Korean J Radiol. 2014; 15:12–19.
12. Kettritz U, Rotter K, Schreer I, Murauer M, Schulz-Wendtland R, Peter D, et al. Stereotactic vacuum-assisted breast biopsy in 2874 patients: a multicenter study. Cancer. 2004; 100:245–251.
13. Zuiani C, Mazzarella F, Londero V, Linda A, Puglisi F, Bazzocchi M. Stereotactic vacuum-assisted breast biopsy: results, follow-up and correlation with radiological suspicion. Radiol Med. 2007; 112:304–317.
14. Golub RM, Bennett CL, Stinson T, Venta L, Morrow M. Cost minimization study of image-guided core biopsy versus surgical excisional biopsy for women with abnormal mammograms. J Clin Oncol. 2004; 22:2430–2437.
15. Hahn SY, Shin JH, Han BK, Ko EY. Sonographically-guided vacuum-assisted biopsy with digital mammography-guided skin marking of suspicious breast microcalcifications: comparison of outcomes with stereotactic biopsy in Asian women. Acta Radiol. 2011; 52:29–34.
16. Jung YJ, Bae YT, Lee JY, Seo HI, Kim JY, Choo KS. Lateral decubitus positioning stereotactic vacuum-assisted breast biopsy with true lateral mammography. J Breast Cancer. 2011; 14:64–68.
17. Burbank F, Parker SH. Methods for Analysis of One-Step Breast Biopsy Programs. Breast J. 1998; 4:307–319.
18. Wunderbaldinger P, Wolf G, Turetschek K, Helbich TH. Comparison of sitting versus prone position for stereotactic large-core breast biopsy in surgically proven lesions. AJR Am J Roentgenol. 2002; 178:1221–1225.
19. Jackman RJ, Rodriguez-Soto J. Breast microcalcifications: retrieval failure at prone stereotactic core and vacuum breast biopsy--frequency, causes, and outcome. Radiology. 2006; 239:61–70.
20. Schrading S, Distelmaier M, Dirrichs T, Detering S, Brolund L, Strobel K, et al. Digital breast tomosynthesis-guided vacuum-assisted breast biopsy: initial experiences and comparison with prone stereotactic vacuum-assisted biopsy. Radiology. 2015; 274:654–662.
21. Welle GJ, Clark M, Loos S, Pauls D, Warden D, Sheffield M, et al. Stereotactic breast biopsy: recumbent biopsy using add-on upright equipment. AJR Am J Roentgenol. 2000; 175:59–63.
22. Jackman RJ, Nowels KW, Rodriguez-Soto J, Marzoni FA Jr, Finkelstein SI, Shepard MJ. Stereotactic, automated, large-core needle biopsy of nonpalpable breast lesions: false-negative and histologic underestimation rates after long-term follow-up. Radiology. 1999; 210:799–805.
23. Lee CH, Philpotts LE, Horvath LJ, Tocino I. Follow-up of breast lesions diagnosed as benign with stereotactic core-needle biopsy: frequency of mammographic change and false-negative rate. Radiology. 1999; 212:189–194.
24. Crystal P, Koretz M, Shcharynsky S, Makarov V, Strano S. Accuracy of sonographically guided 14-gauge core-needle biopsy: results of 715 consecutive breast biopsies with at least two-year follow-up of benign lesions. J Clin Ultrasound. 2005; 33:47–52.
25. Cho N, Moon WK. Digital mammography-guided skin marking for sonographically guided biopsy of suspicious microcalcifications. AJR Am J Roentgenol. 2009; 192:W132–W136.
26. Jackman RJ, Burbank F, Parker SH, Evans WP 3rd, Lechner MC, Richardson TR, et al. Stereotactic breast biopsy of nonpalpable lesions: determinants of ductal carcinoma in situ underestimation rates. Radiology. 2001; 218:497–502.
27. Moon WK, Im JG, Koh YH, Noh DY, Park IA. US of mammographically detected clustered microcalcifications. Radiology. 2000; 217:849–854.
28. Wang LC, Sullivan M, Du H, Feldman MI, Mendelson EB. US appearance of ductal carcinoma in situ. Radiographics. 2013; 33:213–228.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download