Abstract
Objective
To compare the effectiveness of radiofrequency ablation (RFA) combined with transcatheter arterial chemoembolization (TACE) with surgical resection in patients with a single hepatocellular carcinoma (HCC) ranging from 2 to 5 cm.
Materials and Methods
The study participants were enrolled over a period of 29 months and were comprised of 37 patients in a combined therapy group and 47 patients in a surgical resection group. RFA was performed the day after TACE, and surgical resection was performed by open laparotomy. The two groups were compared with respect to the length of hospital stay, rates of major complication, and rates of recurrence-free and overall survival.
Results
Major complications occurred more frequently in the surgical resection group (14.9%) than in the combined therapy group (2.7%). However, there was no statistical significance (p = 0.059). The rates of recurrence-free survival at 1, 2, 3 and 4 years were similar between the combined therapy group (89.2%, 75.2%, 69.4% and 69.4%, respectively) and the surgical resection group (81.8%, 68.5%, 68.5% and 65%, respectively) (p = 0.7962, log-rank test). The overall survival rates at 1, 2, 3 and 4 years were also similar between groups (97.3%, 86.5%, 78.4% and 78.4%, respectively, in the combined therapy group, and 95.7%, 89.4%, 84.3% and 80.3%, respectively, in the surgical resection group) (p = 0.6321, log-rank test).
Although percutaneous radiofrequency ablation (RFA) is effective and safe for treating a small (≤ 3 cm) hepatocellular carcinoma (HCC) (1-4), it could also be a minimally invasive treatment option in patients with larger HCCs (5-7). However, the rate of local tumor progression tends to increase considerably in proportion to the size of the HCC (8). Traditionally, the 3-cm and 5-cm cutoff points are considered important because previous studies showed RFA resulted in a lower complete ablation rate in HCCs larger than these two cutoff points (3, 5, 9, 10).
When compared with RFA alone, the combined therapy of transcatheter arterial chemoembolization (TACE) and RFA has been found more effective, especially for HCCs larger than 3 cm (8, 11-17). Owing to several synergistic effects, RFA combined with TACE could lead to lower local tumor progression rates and an improvement in both recurrence-free and overall survival rates in patients with HCCs. Regarding small HCCs less than 3 cm, RFA alone is similarly effective to the combined therapy (2). However, the combined therapy may be necessary to more effectively block local tumor progression in patients with HCC ranging from 2 cm to 3 cm in size (18).
Although RFA combined with TACE is increasingly used in treating patients with HCCs, there have been relatively few studies on the outcome of this combined treatment in patients with early stage HCC in comparison with surgical resection, in which the disease-free and overall survival rates after treatment with either a combination of RFA and TACE or surgical resection were comparable in patients with early stage HCC (11, 17, 19). Furthermore, in these studies, a considerable proportion of patients (up to 30%) had HCC with diameter less than 2 cm.
Thus, the purpose of our study was to compare the effectiveness of RFA combined with TACE with surgical resection in patients with a single HCC ranging from 2 to 5 cm.
The informed consent for medical procedures was signed by all the patients before treatment. Between August 2008 and December 2010, among 257 consecutive patients who underwent combined treatment with TACE and RFA or surgical resection for the treatment of HCCs, 84 patients who met the following criteria were included in this study: 1) a single HCC ranging between 2 cm and 5 cm in the largest dimension, 2) no history of prior treatment for HCC, 3) no major vascular invasion and 4) no evidence of extrahepatic tumor. All patients were confirmed to have HCC by a percutaneous biopsy (n = 15) or the typical imaging findings (n = 69) according to the American Association for the Study of Liver Disease (20). Any patient who met the following criteria became a potential candidate for the surgical resection: 1) appropriate hepatic functional reserve based on the ICG15 level suggested by Makuuchi et al. (21), 2) the volume of remnant liver after surgical resection should be more than 30% of the total liver volume, 3) bilirubin level less than 2 and 4) no uncontrolled ascites. The final decision between treatment options was made by an institutional hepatobiliary team with the views of patients being taken into account. As a result, among 84 patients, 47 patients were treated with a surgical resection, whereas 37 patients were treated with a combined therapy due to the following reasons: 1) patients wanted to undergo nonsurgical treatment (n = 22), 2) inappropriate hepatic functional reserve (n = 9), 3) small volume of remnant liver after surgery (n = 6). HCCs were divided into two subgroups according to size (≤ 3 cm vs. > 3 cm) and morphology (non-infiltrating vs. infiltrating). Non-infiltrating HCCs were defined as tumor nodules with smooth and well-defined margins surrounded by a capsule (5).
Digital subtraction angiography and subsequent chemoembolization were conducted by an interventional radiologist using a digital subtraction technique (Allura Xper FD20; Philips Medical Systems, Best, Netherlands, or Advantx LCA/LP+; GE Healthcare, Paris, France). After identifying the feeding arteries with common hepatic artery angiography, which supply blood to the hypervascular mass, TACE of the feeding arteries was performed through further super-selective catheterization as close to the tumor as possible. A mixture of doxorubicin hydrochloride (Adriamycin; Ildong Co. Ltd., Seoul, Korea) and an emulsion of iodized oil (Lipiodol; Laboratoire Guerbet, Aulnay Sous Bois, France) was used for chemoembolization. The amount of iodized oil was tailored to the size of the HCC (1 cc per 1-cm diameter of the tumor) with an upper limit of 10 cc. The infusion of the mixture was continued until the flow of the feeding artery was static, and this was followed by the infusion of Gelfoam powder (Cutanplast; Mascia Brunelli, Milan, Italy).
Ultrasound-guided RFA was performed in all the patients one day after TACE. When patients had coagulopathy, considered to be present if the platelet count was less than 50000 mm3 (50 × 109/L) or the international normalized ratio was greater than 1.5, the procedure was performed after platelet transfusion. All patients underwent the procedures under sedation and local anesthesia, and their hemodynamic status was continuously monitored. When performing the insertion of electrodes under ultrasound guidance, intercostal or subcostal approaches were used, depending on the location of the tumor. In cases where the index tumor was located in the vicinity of the diaphragm or the colon, artificial ascites was routinely introduced using normal saline in advance of inserting electrodes under ultrasonographic guidance. Artificial ascites was induced in 14 (38%) of 37 patients to improve the conspicuity of the index tumor or to minimize collateral thermal injury to the adjacent diaphragm or the colon. RFA was performed using a monopolar radiofrequency generator (CC-1, Valleylab, Boulder, CO, USA) with consecutive activation mode and a 17 G dual clustered internally cooled electrode with a 3 cm exposed tip (STARmed Co. Ltd.; Goyang, Korea). When a multiple overlapping insertion of electrodes was required due to the geometry of the tumor, 17 G dual clustered, 17 G single and/or 15 G single internally cooled electrode with 3 cm exposed tip (STARmed Co. Ltd.; Goyang, Korea) were additionally used. The number of used applicators ranged between one and three (one, two and three electrodes in 15, 17 and 5 patients, respectively). That is, one 17 G dual clustered electrode was used in 15 patients, whereas two or three electrodes (a combination of dual clustered electrodes, 7 patients; a combination of dual clustered and single electrodes, 15 patients) were used in the remaining 22 patients. In general, one 17 G dual clustered electrode approach was preferentially used for the index tumor less than 3.5 cm. Radiofrequency current was emitted with the generator set to deliver the maximal power in the automatic impedance control mode. To maintain the temperature of the electrode tip below 20℃, iced physiologic saline was continuously circulated through the cooling catheter connected to the electrode by a peristaltic pump (Watson Marlow; Wilmington, MA, USA). Tumor ablation was continued for 8 to 12 minutes at each electrode placement. RFA was finished when the visible tumor was fully covered by a transient hyperechoic ablated zone on ultrasound images. To prevent bleeding or tumoral seeding, the electrode track was ablated while retracting the electrode.
All procedures were performed by open laparotomy. The type of hepatic resection was based on the size and location of the tumor (subsegmentectomy, n = 3; monosegmentectomy, n = 14; bisegmentectomy, n = 16; lobectomy, n = 14). All surgical resections were finished after negative resection margins were confirmed by histopathological evaluation.
A contrast-enhanced CT was performed within three hours of RFA. When tumoral enhancement around the RF ablation zone was detected, an additional session of RFA was performed for the residual tumor. The technical effectiveness of the combined therapy was defined by the absence of an enhanced tumor area, as assessed on one-month follow-up CT (22). All the patients in both groups underwent follow-up CT every three months for two years and every six months after two years. Physical examination and blood tests, including a serum a-fetoprotein and liver function test, were also performed at each visit. Local tumor progression was defined as a newly appearing tumoral enhancement in or along the margin of the ablated zone in the combined therapy group and around the resection margin in the surgical resection group (17). In cases where extrahepatic metastases were suspected, PET/CT and bone scintigraphy were also performed. Once local tumor progression and/or new lesions in the liver were detected during follow-up, the patients were treated using various treatment options, including RFA, TACE and systemic chemotherapy.
The two groups were compared with respect to the length of hospital stay, rates of major complication, rates of recurrence-free survival and overall survival rates. Major complications were considered to be present if there was any event that resulted in permanent adverse sequelae (including substantial morbidity and disability), required additional treatment (including increased level of care), or led to increased hospital stay or hospital re-admission (22). Post-procedural pain and fever of unknown origin were not considered major complications. For rates of recurrence-free survival, the occurrence of local tumor progression, an intrahepatic new lesion or a distant metastasis during the time between the treatment and the last follow-up visit was used. For overall survival rates, the time between the treatment and death or the last follow-up visit was used.
Statistical analyses were performed using MedCalc Version 12.3 computer software (MedCalc, Mariakerke, Belgium). The two groups were compared on the independent continuous variables and categorical variables using Student's t test and the chi-square test, respectively. Rates of recurrence-free survival and overall survival rates were estimated using the Kaplan-Meier method. The rates for the two groups were compared using the log-rank test. The prognostic significance of baseline characteristics was evaluated using multivariate Cox proportional hazards models. Null hypotheses of no difference were rejected if p-values were less than 0.05.
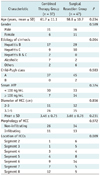
The demographic and clinical characteristics of both groups are summarized in Table 1. There were no significant differences between groups of age, gender, etiology of cirrhosis, Child-Pugh class, serum alpha-fetoprotein (AFP) level, tumor size, morphology or location of the HCCs.
In the combined therapy group, the index tumor was ablated completely in all 37 patients on immediate CT after ablation so that no additional sessions of RFA were required. Furthermore, the technical effectiveness was noted in all 37 patients on one-month follow-up CT (Fig. 1). The mean ablation time was 27.3 ± 13.3 minutes. The mean number of ablations per each ablation session was 2.6 ± 1.5. Regarding the surgical resections, all procedures were curatively performed.
The length of hospital stay after the procedure was significantly longer in the surgical resection group (19.8 ± 8.4 days) than in the combined therapy group (7.4 ± 2.2 days) (p < 0.0001). Major complications occurred in one (2.7%) of the 37 patients in the combined therapy group and in 7 (14.9%) of the 47 patients in the surgical resection group (p = 0.059). In the combined therapy group, one patient showed segmental dilatation of the intrahepatic bile duct on a follow-up CT due to the injury of bile duct. The surgical resection group showed the following complications: pleural effusion (n = 2), which required chest tube drainage due to dyspnea, aspiration pneumonia (n = 2), hepatic failure (n = 1), hepatic abscess (n = 1) and mechanical ileus (n = 1). All of the patients recovered from complications with conservative treatment and/or percutaneous intervention, except one patient who died as a result of progression of hepatic failure after right hepatectomy.
Patients were followed up for 1-49.8 months. The mean (± standard deviation) follow-up time for the combined therapy group was 29.9 months (± 7.8) and for the surgical resection group 31.7 months (± 10). During the follow-up period in the combined therapy group, local tumor progression was detected in 2, intrahepatic new lesions in 5, and distant metastasis in 4 patients (Table 2). One patient had both local tumor progression and distant metastasis to the lung. The rate of local tumor progression at 1 year was 2.7%, at 2 years 5.4%, 3 years 5.4% and 4 years 5.4%. In the surgical resection group, local tumor progression was observed in three patients and an intrahepatic new lesion in 10 patients. Distant metastasis occurred in two patients (Table 2). One patient had both intrahepatic new lesion and distant metastasis to the lung. In each treatment group, there were no significant differences between patients with small (2-3 cm) and medium (3.1-5 cm) HCC in terms of the incidence of local tumor progression and overall recurrence.
The rates of recurrence-free survival at 1, 2, 3 and 4 years were 89.2%, 75.2%, 69.4% and 69.4%, respectively, in the combined therapy group and 81.8%, 68.5%, 68.5% and 65%, respectively, in the surgical resection group (Fig. 2). There was no significant difference between the two groups (p = 0.7962, log-rank test). Also, there were no significant differences between the two groups for patients with small HCC (p = 0.9599, log-rank test) and medium HCC (p = 0.7783, log-rank test). Among various factors that included age, sex, location, size and morphology of HCC, liver cirrhosis (LC) cause, serum AFP level, Child-Pugh class, treatment allocation, multivariate Cox proportional hazards regression model revealed that serum AFP level was a significant independent factor associated with recurrence (odds ratio, 1.0002; 95% confidence interval, 1.0000-1.0004; p = 0.0246).
During follow-up period, seven patients in the combined therapy group died due to hepatic failure (n = 4), hemoptysis (n = 2) or variceal bleeding (n = 1). In the surgical resection group, eight patients died due to hepatic failure (n = 7) or gastrointestinal bleeding (n = 1). The overall survival rates at 1, 2, 3 and 4 years were 97.3%, 86.5%, 78.4% and 78.4%, respectively, in the combined therapy group, and 95.7%, 89.4%, 84.3% and 80.3%, respectively, in the surgical resection group (Fig. 3). No significant difference was found between the two groups (p = 0.6321, log-rank test). Also, there were no significant differences between the two groups for patients with small HCC (p = 0.5618, log-rank test) and medium HCC (p = 0.3216, log-rank test). Among various factors that included age, sex, location, size and morphology of HCC, LC cause, serum AFP level, Child-Pugh class, treatment allocation, there were no significant predictors affecting overall survival on multivariate Cox proportional hazards regression model.
Local tumor progression after RFA is closely correlated with the occurrence of new HCCs in previously untreated liver through microscopic vascular invasion or satellite tumor nests (2, 13, 23). Successful local tumor control is therefore an important prognostic factor for good clinical outcomes in patients with HCCs treated by local ablation therapy (2, 24).
There are a number of techniques for achieving a large ablated zone and complete necrosis of HCCs (2). Among these procedures, combined TACE and RFA, as opposed to RFA alone, has several advantages for attaining better local tumor control. The decreased arterial blood flow to an HCC induced by TACE may reduce the heat sink effect of large vessels adjacent to HCC, resulting in considerable increase in the volume of the ablation zone by RFA (2, 11, 15, 16, 25). In addition, the effect of chemotherapy and hypoxic injury induced by TACE on cancer cells is enhanced by the high temperature during RF thermal ablation, making it possible to extend the ablated zone (11, 26, 27). Given that the incomplete ablation and/or considerable local tumor progression limits the effectiveness of RFA alone, RFA combined with TACE was expected to be more effective especially for HCCs greater than 3 cm. Indeed, combined therapy is superior to RFA alone in providing a better local control of HCCs larger than 3 cm (5, 8, 13, 15, 17).
Radiofrequency ablation alone achieves complete necrosis in more than 90% of small (≤ 3 cm) HCCs (3). Thus, RFA alone seems to be sufficiently effective for the treatment of HCCs smaller than 3 cm (2). However, residual viable tumor cells that could not be detected on contrast-enhanced CT might exist and eventually cause local tumor progression during the follow-up period after the procedure (2). In fact, Komorizono et al. (28) reported that a tumor size larger than 2 cm was one of the risk factors for local recurrence. Further, according to Mazzaferro et al. (29), viable microscopic tumor tissue was present after RFA treatment in 37% of patients with HCCs less than 3 cm and in 71% with HCCs larger than 3 cm. Combined therapy was recently found better than RFA alone with respect to local tumor control in the treatment of HCC (2-3 cm in diameter) (18). Thus, among small (≤ 3 cm) HCCs, HCCs ranging between 2 cm and 3 cm were treated with the combined therapy in our study.
Given that an insufficient ablative margin is associated with the occurrence of local tumor progression and that an ablative margin of at least 3 mm is required to reduce the rate of local tumor progression after RFA (30), a single needle-insertion session of RFA could be insufficient for the treatment of HCCs larger than 2 cm in the longest diameter. When the HCC is larger than 2 cm, a multiple overlapping ablation technique frequently needs to be performed in order to achieve the complete necrosis of an HCC surrounded by an optimal ablative margin. Even with multiple overlapping ablations for medium HCC, the complete ablation rate was lower compared to small HCC (31). In our study, when a multiple overlapping technique was needed, multiple electrodes including at least one cluster-type electrode were inserted in the index tumor and activated consecutively. This was because multiple electrode approach was considered to overcome the technical difficulty of repositioning of the single electrode under ultrasound guidance due to the gas bubbles (32). The use of multiple active electrodes was also expected to be superior to conventional overlapping technique with a single electrode (33). The technical effectiveness of ultrasound-guided RFA ranges from 92.9% to 100% in medium HCCs (32, 33). In addition, ultrasound-guided RFA with a cluster electrode and multiple electrode approach resulted in better local therapeutic efficacy than multiple overlapping ablations with the same electrode. In our study, technical effectiveness was achieved in all 37 (100%) patients, comparable with the results of previous studies (32, 33). This favorable result might be attributable to the use of combination therapy and multiple electrode approach including at least one cluster electrode.
When performing RFA under ultrasound guidance, precise targeting of the index tumor may be difficult due to the tumor location. For example, if the tumor is located in the liver dome and adjacent to the diaphragm, this tumor frequently has limited visibility due to overlapped lung or ribs (34). Artificial ascites method was effectively applied in this setting (35). Thus, in our study, artificial ascites method was used in 38% of the combined group to improve ultrasound-guided targeting of HCC on ultrasound images.
Regarding the sequencing of TACE and RFA in the combined therapy, it seems reasonable to perform TACE first in order to take full advantage of the synergistic effects of TACE upon RFA (17). There might be concern that the efficacy of chemotherapeutic agents infused during TACE could be reduced if they were exposed to hyperthermia during RFA (36). However, when the degree and duration of the high temperature generated by a RF system are taken into consideration, the cytotoxic potency of anticancer drugs appears not to be appreciably affected (27).
There has been no obvious consensus regarding the optimal interval between TACE and RFA with previous studies adopting different intervals, ranging from the same day to two months (2, 8, 11-13, 15). Theoretically, embolization effect that results from TACE may be maximal when the combined therapy is performed on the same day (15). However, an experimental study revealed that RFA performed within 6 days of TACE resulted in larger ablation volumes as compared to RFA alone (37). In our study, RFA was performed approximately 24 hours after TACE. This was because a one-day interval between modalities could strike a balance among the several concerns about the risk of complications, such as hepatic failure or hepatic infarction, the potential negative effect of hyperthermia on chemotherapy and a reduction in the effect of chemoembolization proportional to elapsed time from when TACE was performed (15).
In our study, there was no significant difference between groups with regard to recurrence-free survival rates, agreeing with previous studies (11, 17). Several studies evaluating RFA combined with TACE for HCC smaller than 5 cm demonstrated the rates of local tumor progression from 2.9% to 40% (8, 13, 15, 17). In our study, local tumor progression was noted in two (5.4%) patients. Although our result was better than those of previous reports, it was difficult to directly compare our study result with those of previous studies due to the different basic values, such as method of combined therapy, type of electrode used, mean tumor size, follow-up duration. In our study, serum AFP level was found to be a significant independent factor associated with recurrence. Indeed, patients with high serum AFP level are expected to develop recurrence more frequently (31), resulting in a worse prognosis. Thus, based on our study, after complete resection or combined therapy of the primary tumor, close follow-up seems to be needed especially in patients with AFP level greater than 100 ng/mL.
As compared with small HCC, medium HCC was reported to be significantly correlated with a higher local tumor progression after combined therapy (8). Further, during follow-up period after the combined therapy, local tumor progression was more frequently observed in patients with medium HCC (40%) than HCC between 2 and 3 cm in diameter (16%) (13, 18). Conversely, in our study, local tumor progression was observed in one (9.1%) and one (3.8%) of patients with medium HCC and HCC between 2 and 3 cm in diameter, respectively. We assume that this discrepancy was partly due to small size of patients with HCC between 2 and 3 cm and different number of electrodes used in medium HCC and HCC between 2 and 3 cm.
In terms of overall survival rates, surgical resection has been considered to be the best treatment for patients with resectable HCCs and reserved hepatic function, and is regarded as the first-line treatment, especially for HCCs larger than 3 cm (15, 38). In our study, the overall survival rates at 1, 2, 3 and 4 years were similar between the combined therapy group and the surgical resection group, comparable with the results of previous studies (11, 17, 19). However, the overall survival rates in our study were similar to or lower than others (11, 17). This discordance could be associated with a difference in the mean size of the HCCs (larger than 3 cm in our study vs. smaller than 3 cm in those two studies).
In comparison to liver resection, our study revealed that the combined therapy resulted in a shorter length of hospitalization. Further, although there was no significant difference, we observed fewer major complications and no procedure-related death in the combined therapy group. These findings are consistent with those of other studies (11, 17, 19). Thus, we believe that RFA combined with TACE was shown to be minimally invasive and safe in treating patients with early stage HCCs.
This study has several limitations. First, histologic confirmation of HCCs was not obtained in every patient. Second, our study included a relatively small number of patients and a short follow-up period. Third, there may be an inherent selection bias because this study was retrospective and nonrandomized in design. Thus, both patient groups were not homogeneous for sample size. Although a randomized controlled study would be more desirable, it would be hard to conduct due to ethical considerations.
In conclusion, when compared with surgical resection for the treatment of a single HCC ranging from 2 to 5 cm, RFA combined with TACE showed similar results in terms of recurrence-free and overall survival rates.
Figures and Tables
Fig. 1
Images of 59-year-old man with 5 cm non-infiltrating HCC, who underwent combined TACE and RFA.
A. Fat-suppressed T2-weighted axial MR image obtained 1 week before combined treatment of TACE and RFA shows 5 cm heterogeneous hyperintense mass (arrowheads) in right hepatic lobe. B. Post-TACE angiogram shows dense radiopaque mass (arrows) with accumulated iodized oil in right hepatic lobe. C. US image during RFA shows mass surrounded by transient hyperechoic zone (arrowheads) and echogenic RF electrode (arrow) within mass. D. Contrast-enhanced CT image obtained 24 months after combined therapy shows dense iodized oil accumulation in mass (asterisk) surrounded by RF-induced coagulation (arrowheads), without local tumor progression. HCC = hepatocellular carcinoma, TACE = transcatheter arterial chemoembolization, RFA = radiofrequency ablation, US = ultrasonography

Fig. 2
Graph illustrates recurrence-free survival rates in patients with single hepatocellular carcinoma ranging from 2 to 5 cm treated with combined therapy or surgical resection.
There was no significant difference between two groups (p = 0.7962, log-rank test).

Fig. 3
Graph shows overall survival rates in patients with single hepatocellular carcinoma ranging from 2 to 5 cm treated with combined therapy or surgical resection. No significant difference was seen between two groups (p = 0.6321, log-rank test).

References
1. Curley SA. Radiofrequency ablation of malignant liver tumors. Ann Surg Oncol. 2003; 10:338–347.
2. Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009; 252:905–913.
3. Shibata T, Shibata T, Maetani Y, Isoda H, Hiraoka M. Radiofrequency ablation for small hepatocellular carcinoma: prospective comparison of internally cooled electrode and expandable electrode. Radiology. 2006; 238:346–353.
4. Sutherland LM, Williams JA, Padbury RT, Gotley DC, Stokes B, Maddern GJ. Radiofrequency ablation of liver tumors: a systematic review. Arch Surg. 2006; 141:181–190.
5. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000; 214:761–768.
6. Seror O, N'Kontchou G, Ibraheem M, Ajavon Y, Barrucand C, Ganne N, et al. Large (>or=5.0-cm) HCCs: multipolar RF ablation with three internally cooled bipolar electrodes--initial experience in 26 patients. Radiology. 2008; 248:288–296.
7. Yin XY, Xie XY, Lu MD, Xu HX, Xu ZF, Kuang M, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009; 115:1914–1923.
8. Takaki H, Yamakado K, Nakatsuka A, Fuke H, Murata K, Shiraki K, et al. Radiofrequency ablation combined with chemoembolization for the treatment of hepatocellular carcinomas 5 cm or smaller: risk factors for local tumor progression. J Vasc Interv Radiol. 2007; 18:856–861.
9. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999; 210:655–661.
10. Morimoto M, Sugimori K, Shirato K, Kokawa A, Tomita N, Saito T, et al. Treatment of hepatocellular carcinoma with radiofrequency ablation: radiologic-histologic correlation during follow-up periods. Hepatology. 2002; 35:1467–1475.
11. Kagawa T, Koizumi J, Kojima S, Nagata N, Numata M, Watanabe N, et al. Transcatheter arterial chemoembolization plus radiofrequency ablation therapy for early stage hepatocellular carcinoma: comparison with surgical resection. Cancer. 2010; 116:3638–3644.
12. Kang SG, Yoon CJ, Jeong SH, Kim JW, Lee SH, Lee KH, et al. Single-session combined therapy with chemoembolization and radiofrequency ablation in hepatocellular carcinoma less than or equal to 5 cm: a preliminary study. J Vasc Interv Radiol. 2009; 20:1570–1577.
13. Kim JH, Won HJ, Shin YM, Kim SH, Yoon HK, Sung KB, et al. Medium-sized (3.1-5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol. 2011; 18:1624–1629.
14. Maluccio M, Covey AM, Gandhi R, Gonen M, Getrajdman GI, Brody LA, et al. Comparison of survival rates after bland arterial embolization and ablation versus surgical resection for treating solitary hepatocellular carcinoma up to 7 cm. J Vasc Interv Radiol. 2005; 16:955–961.
15. Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010; 116:5452–5460.
16. Rossi S, Garbagnati F, Lencioni R, Allgaier HP, Marchianò A, Fornari F, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000; 217:119–126.
17. Yamakado K, Nakatsuka A, Takaki H, Yokoi H, Usui M, Sakurai H, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology. 2008; 247:260–266.
18. Kim JW, Kim JH, Won HJ, Shin YM, Yoon HK, Sung KB, et al. Hepatocellular carcinomas 2-3 cm in diameter: transarterial chemoembolization plus radiofrequency ablation vs. radiofrequency ablation alone. Eur J Radiol. 2012; 81:e189–e193.
19. Helmberger T, Dogan S, Straub G, Schrader A, Jüngst C, Reiser M, et al. Liver resection or combined chemoembolization and radiofrequency ablation improve survival in patients with hepatocellular carcinoma. Digestion. 2007; 75:104–112.
20. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022.
21. Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993; 9:298–304.
22. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009; 20:7 Suppl. S377–S390.
23. Okada S, Shimada K, Yamamoto J, Takayama T, Kosuge T, Yamasaki S, et al. Predictive factors for postoperative recurrence of hepatocellular carcinoma. Gastroenterology. 1994; 106:1618–1624.
24. Takahashi S, Kudo M, Chung H, Inoue T, Ishikawa E, Kitai S, et al. Initial treatment response is essential to improve survival in patients with hepatocellular carcinoma who underwent curative radiofrequency ablation therapy. Oncology. 2007; 72:Suppl 1. 98–103.
25. Buscarini E, Savoia A, Brambilla G, Menozzi F, Reduzzi L, Strobel D, et al. Radiofrequency thermal ablation of liver tumors. Eur Radiol. 2005; 15:884–894.
26. Goldberg SN, Girnan GD, Lukyanov AN, Ahmed M, Monsky WL, Gazelle GS, et al. Percutaneous tumor ablation: increased necrosis with combined radio-frequency ablation and intravenous liposomal doxorubicin in a rat breast tumor model. Radiology. 2002; 222:797–804.
27. Veltri A, Moretto P, Doriguzzi A, Pagano E, Carrara G, Gandini G. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC). Eur Radiol. 2006; 16:661–669.
28. Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003; 97:1253–1262.
29. Mazzaferro V, Battiston C, Perrone S, Pulvirenti A, Regalia E, Romito R, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004; 240:900–909.
30. Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010; 195:758–765.
31. Lam VW, Ng KK, Chok KS, Cheung TT, Yuen J, Tung H, et al. Incomplete ablation after radiofrequency ablation of hepatocellular carcinoma: analysis of risk factors and prognostic factors. Ann Surg Oncol. 2008; 15:782–790.
32. Park MJ, Kim YS, Rhim H, Lim HK, Lee MW, Choi D. A comparison of US-guided percutaneous radiofrequency ablation of medium-sized hepatocellular carcinoma with a cluster electrode or a single electrode with a multiple overlapping ablation technique. J Vasc Interv Radiol. 2011; 22:771–779.
33. Lee J, Lee JM, Yoon JH, Lee JY, Kim SH, Lee JE, et al. Percutaneous radiofrequency ablation with multiple electrodes for medium-sized hepatocellular carcinomas. Korean J Radiol. 2012; 13:34–43.
34. Kang TW, Rhim H, Kim EY, Kim YS, Choi D, Lee WJ, et al. Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: assessment of safety and therapeutic efficacy. Korean J Radiol. 2009; 10:34–42.
35. Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol. 2008; 190:91–98.
36. Ahrar K, Newman RA, Pang J, Vijjeswarapu MK, Wallace MJ, Wright KC. 2004 Dr. Gary J. Becker Young Investigator Award: Relative thermosensitivity of cytotoxic drugs used in transcatheter arterial chemoembolization. J Vasc Interv Radiol. 2004; 15:901–905.
37. Guang C, Kawai N, Sato M, Takasaka I, Minamiguchi H, Sahara S, et al. Effect of interval between transcatheter hepatic arterial embolization and radiofrequency ablation on ablated lesion size in a swine model. Jpn J Radiol. 2011; 29:649–655.
38. Lupo L, Panzera P, Giannelli G, Memeo M, Gentile A, Memeo V. Single hepatocellular carcinoma ranging from 3 to 5 cm: radiofrequency ablation or resection? HPB (Oxford). 2007; 9:429–434.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download