Abstract
We present a case of pulmonary vein (PV) stenosis after radio-frequency (RF) ablation, in which a hemodynamic change in the pulmonary artery was similar to that of congenital PV atresia on time-resolved contrast-enhanced magnetic resonance angiography (TR-MRA). A 48-year-old man underwent RF ablation due to atrial fibrillation. The patient subsequently complained of hemoptysis, dyspnea on exertion, and right chest pain. Right PV stenosis after catheter ablation was diagnosed through chest computed tomography and lung perfusion scan. Pulmonary TR-MRA revealed the pulmonary artery via systemic arterial collaterals and draining systemic collateral veins. On a velocity-encoded cine image, the flow direction of the right pulmonary artery was reversed in the diastolic phase and the left pulmonary artery demonstrated continuous forward flow throughout the cardiac cycle. These hemodynamic changes were similar to those seen in congenital unilateral PV atresia.
Pulmonary vein (PV) stenosis is a recognized potential complication of radiofrequency (RF) ablation of atrial fibrillation (AF). PV stenosis acquired after RF ablation varies in severity from asymptomatic to hemoptysis or exertional dyspnea. PV stenosis acquired after RF ablation is mainly diagnosed by computed tomography (CT) angiography, magnetic resonance (MR) angiography, and conventional angiography, and is treated through balloon angioplasty and stent implantation (1). However, symptoms usually improve spontaneously over time with improvement in the radiologic abnormality, and hemodynamic compensatory mechanisms may play a role in this (2). However, few studies have been conducted to examine how rapidly these hemodynamic changes occur and their precise mechanisms.
Time-resolved (four-dimensional) contrast-enhanced MR angiography (TR-MRA), which involves the acquisition of angiographic images at different times, is useful in understanding the hemodynamics of blood vessels (3, 4).
We report a case of compensatory hemodynamic change that occurred following RF ablation in a patient with PV stenosis on TR-MRA and describe its similar mechanics to those of congenital PV atresia.
A 48-year-old man underwent RF ablation due to AF. Since then, the patient complained of intermittent hemoptysis and dyspnea upon exertion. However, symptoms were transient and improved after conservative treatment. The patient was re-admitted 6 months after the procedure because of an 1-month history of right chest pain.
The chest posteroanterior view at admission showed right pleural effusion and patchy consolidations in the right lower lung field. Smooth reticular opacities overlapped and the volume of the right lung was smaller than that of the left side. Comparison with a prior chest radiography taken before RF ablation revealed that the lesions were newly developed.
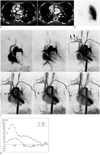
Chest CT showed ill-defined soft tissue infiltrations in the right mediastinum, which encased the bronchovascular bundle. The right superior PV was faintly opacified and disconnected. The right inferior PV was not opacified and its entry to the left atrium was obliterated by the soft tissue infiltrations. Small collateral vessels were noted around the soft tissue infiltrations (Fig. 1A). Pulmonary vein stenosis after catheter ablation was diagnosed. In the lung setting, parenchymal pulmonary venous infarction was also noted. A lung perfusion scan revealed the absence of right lung perfusion (Fig. 1B). For the evaluation of pulmonary vein flow, pulmonary TR-MRA (TR = 2.7 ms, TE = 1.0 ms, flip angle = 19°, a sample interval = 1 sec) was performed using a 3 Tesla system (Magnetom VERIO, Siemens Healthcare, Erlangen, Germany). On pulmonary TR-MRA, the right pulmonary artery was shown to be opacified later than the left pulmonary artery. Prominent systemic arteries were also noted in the right thorax that connected with the right pulmonary artery, suggesting pulmonary to systemic collaterals. Systemic venous collaterals were opacified instead of filling the pulmonary vein (Fig. 1C). On a velocity-encoded cine (VEC) image (TR = 38.2 ms, TE = 2.3 ms, signal average 1, matrix size = 256 × 256, FOV = 320 × 320 mm, slice thickness = 6 mm, flip angle = 20°, velocity encoding range -100 to 100 cm/sec), the flow direction of the right pulmonary artery was reversed in the diastolic phase and displayed a continuous diastolic forward flow in the left pulmonary artery (Fig. 1D), which was similar to that of congenital PV atresia.
An attempt to insert a stent into the pulmonary vein was unsuccessful. However, symptoms and abnormalities on chest PA improved after heparin anticoagulation therapy. The patient was discharged and followed-up at an outpatient clinic.
Pulmonary vein has been identified as the source of triggers that initiate AF (5). Catheter ablation of AF is effective in approximately 80% of patients, with about 70% not requiring further antiarrhythmic drugs during intermediate follow-up. Major complications that occur in a small number of cases (6%) include procedure-related deaths, strokes, transient ischemic attacks, tamponade and PV stenosis (6). The incidence of PV stenosis has been reported to range from 3% to 42%, depending on the ablative technique used and the method of assessment (2). The incidence has fallen with improvements in the technique; nevertheless, in the updated worldwide registry, significant (> 50%) stenosis accounts for approximately 30% of major complications (6). Thus, PV stenosis represents a relatively common complication.
The spectrum of symptoms caused by postablation PV stenosis is characterized by various symptoms ranging from persistent cough, to significant hemoptysis, and severe exertional dyspnea. Due to these nonspecific symptoms, some patients require additional treatment after being misdiagnosed with other diseases such as pneumonia or pulmonary embolism (2). In our case, the diagnosis of PV stenosis was delayed due to intermittent, transient symptoms with improvement after conservative therapy.
There is variability in the treatment for PV stenosis acquired after RF ablation. In a large worldwide survey, about 22% of cases of severe stenosis (> 50%) that required a PV corrective intervention (6). In cases where an intervention was necessary, stent implantation shows better medium-term prognosis than balloon angioplasty because in more than 50% of patients who undergo a balloon angioplasty, re-stenosis occurs within a year (1). However, in most cases of mild to moderate PV stenosis, even cases of severe PV stenosis, sign and symptoms improve spontaneously. This might be due to the improvement of stenosis and the compensatory hemodynamics (2).
Little is known about how rapidly these compensatory hemodynamic changes occur, or the exact mechanisms by which they occur. Part of the lack of information is due to PV stenosis cannot be demonstrated by imaging. The reports available suggest that a lack of perfusion on a lung perfusion scan is indicative of pulmonary artery to systemic collaterals, and this correlates well with a MR perfusion image (7).
In the current case, TR-MRA demonstrated compensatory hemodynamic changes in PV stenosis. TR-MRA involves rapid sequential imaging of an anatomic volume during the dynamic intravascular passage of a contrast bolus. Imaging begins before the arrival of the contrast agent and continues for as many volume measurements as required to suit the clinical application. As a result, arterial and venous phases of luminal opacification are clearly discriminated. This approach enables the identification and evaluation of transient vascular phenomena or complex vascular flow kinetics (4). Steady technologic advances, including ultrafast pulse sequences, phased multi-array surface coils, parallel data acquisition techniques, and the widespread availability of high-field magnetic resonance systems, have enhanced the clinical usefulness of TR-MRA (3, 4). In our case, TR-MRA directly demonstrated pulmonary to systemic collaterals. In addition to TR-MRA, VEC revealed the reversed flow direction of the right pulmonary artery in the diastolic phase and continuous diastolic forward flow of the left pulmonary artery. Such hemodynamic changes are similar to those apparent in congenital PV atresia. In congenital unilateral PV atresia, pulmonary capillary wedge pressure is increased due to increased venous pressure, and retrograde flow of the ipsilateral pulmonary artery is noted due to systemic to pulmonary arterial collaterals. Oxygen saturation in the affected pulmonary artery is also increased. The resulting perfusion of the affected lung is absent on a perfusion scan. Reversed pulmonary flow is directed to the contralateral pulmonary artery, which leads to the continuous forward flow on the VEC. Peripheral pulmonary veins drain into various collaterals (8, 9). It is conceivable that it underwent a series of temporally-rapid adaptations beginning from pulmonary vein occlusion and consequent development of systemic arterial collateral, resulting in the drainage of oxygen-rich blood back to the contralateral pulmonary artery.
In conclusion, we present a case of PV stenosis after RF ablation, showing hemodynamic change after PV stenosis similar to that of congenital PV atresia on TR-MRA.
Figures and Tables
Fig. 1
Pulmonary vein stenosis after radiofrequency ablation in a 48-year-old man.
A. Chest CT with mediastinal setting shows ill-defined soft tissue infiltrations in right mediastinum encases bronchovascular bundle. Right superior pulmonary vein is faintly opacified and disconnected (thick arrows), and right inferior pulmonary vein is obstructed (thin arrow). B. Lung perfusion scan with Tc-99m MAA shows absence of right lung perfusion. C. Coronal MIP images from 3D time-resolved MR angiography (TR/TE = 2.7/1.0 ms, flip angle = 19°), with sample interval of 1 second, shows later opacification of right pulmonary artery, relative to left pulmonary artery. Note early opacification of right chest wall vein (arrows), and connection between pulmonary artery and systemic arteries (arrowheads). MIP = maximum intensity projection. D. Time-flow volume curves show reversed flow direction of right pulmonary artery (RPA) in diastolic phase and continuous diastolic forward flow in left pulmonary artery (LPA).
References
1. Latson LA, Prieto LR. Congenital and acquired pulmonary vein stenosis. Circulation. 2007. 115:103–108.
2. Saad EB, Marrouche NF, Saad CP, Ha E, Bash D, White RD, et al. Pulmonary vein stenosis after catheter ablation of atrial fibrillation: emergence of a new clinical syndrome. Ann Intern Med. 2003. 138:634–638.
3. Griffin M, Grist TM, François CJ. Dynamic four-dimensional MR angiography of the chest and abdomen. Magn Reson Imaging Clin N Am. 2009. 17:77–90.
4. Lohan DG, Krishnam M, Tomasian A, Saleh R, Finn JP. Time-resolved MR angiography of the thorax. Magn Reson Imaging Clin N Am. 2008. 16:235–248. viii
5. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998. 339:659–666.
6. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010. 3:32–38.
7. Kluge A, Dill T, Ekinci O, Hansel J, Hamm C, Pitschner HF, et al. Decreased pulmonary perfusion in pulmonary vein stenosis after radiofrequency ablation: assessment with dynamic magnetic resonance perfusion imaging. Chest. 2004. 126:428–437.
8. Heyneman LE, Nolan RL, Harrison JK, McAdams HP. Congenital unilateral pulmonary vein atresia: radiologic findings in three adult patients. AJR Am J Roentgenol. 2001. 177:681–685.
9. Roman KS, Kellenberger CJ, Macgowan CK, Coles J, Redington AN, Benson LN, et al. How is pulmonary arterial blood flow affected by pulmonary venous obstruction in children? A phase-contrast magnetic resonance study. Pediatr Radiol. 2005. 35:580–586.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download