Abstract
Objective
To evaluate the prognostic value of volume-based metabolic parameters measured with 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) in patients with clinically node-negative (cN0) oral tongue squamous cell carcinoma (OTSCC) as compared with other prognostic factors.
Materials and Methods
In this study, we included a total of 57 patients who had been diagnosed with cN0 tongue cancer by radiologic, 18F-FDG PET/CT, and physical examinations. The maximum standardized uptake value (SUVmax), average SUV (SUVavg), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) for primary tumors were measured with 18F-FDG PET. The prognostic significances of these parameters and other clinical variables were assessed by Cox proportional hazards regression analysis.
Results
In the univariate analysis, pathological node (pN) stage, American Joint Committee on Cancer (AJCC) stage, SUVmax, SUVavg, MTV, and TLG were significant predictors for survival. On a multivariate analysis, pN stage (hazard ratio = 10.555, p = 0.049), AJCC stage (hazard ratio = 13.220, p = 0.045), and MTV (hazard ratio = 2.698, p = 0.033) were significant prognostic factors in cN0 OTSCC patients. The patients with MTV ≥ 7.78 cm3 showed a worse prognosis than those with MTV < 7.78 cm3 (p = 0.037).
Cancer of the head and neck is the sixth most common malignancy worldwide (1, 2). Among these cancer types, oral tongue squamous cell carcinoma (OTSCC) is the most common cancer diagnosed in the oral cavity and is more frequently associated with metastasis to regional lymph nodes than any other cancer of the oral cavity due to a rich lymphatic network and highly muscularized structure (3). Thus, the clinical course of OTSCC is frequently difficult to predict because the patients with small primary tongue lesions without clinical evidence of metastasis show a relatively high incidence of occult metastasis (4). It has been reported that patients with OTSCC have a significantly worse prognosis than those with similar lesions of the head and neck malignancy such as the oropharynx, larynx, hypopharynx, and other oral cavity sites (1, 5). With increasing evidence demonstrating that OTSCC is biologically different from other head and neck squamous cell carcinomas (HNSCC), the need for prognostication studies to separate OTSCC from HNSCC have emerged (5, 6). There are several studies to evaluate prognostic factors for OTSCC using histopathologic results and molecular markers (1, 2); however, the data are conflicting and it is difficult to use such factors for pretreatment decision-making because they can only be precisely determined in postsurgical pathologic specimens. Therefore, data from prognostication studies specific to OTSCC at the time of initial diagnosis are still limited.
During the last decade, 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) has emerged as an essential imaging tool in the field of oncology (7-10). 18F-FDG PET is useful in detecting cervical lymph node metastasis in HNSCC (11, 12); however, micrometastases still go undetected. In clinically node-negative (cN0) patients with OTSCC, the use of neck dissection in surgical management has been a source of debate, mainly because of a relatively high rate of occult metastases (13, 14).
18F-fluorodeoxyglucose PET has been widely used, not only to determine stage, but also to predict the prognosis in patients with HNSCC. In the past, several studies have suggested that the degree of 18F-FDG uptake, quantified as the maximum standardized uptake value (SUVmax), is an independent prognostic factor predicting clinical outcome in HNSCC (15, 16). Recently, volumetric parameters measured by 18F-FDG PET have emerged as new prognostic factors. Metabolic tumor volume (MTV) is defined as the volume of 18F-FDG activity in the tumor, and total lesion glycolysis (TLG) is a sum of SUV within the tumor. These factors have been reported as significant prognostic factors in several malignancies including instances where HNSCC is better than SUVmax (17-19). In our previous study evaluating 43 patients with cN0 OTSCC, MTV determined by PET/CT, demonstrated a statistically significant correlation with occult nodal metastasis (20). However, to the best of our knowledge, there are no previous studies evaluating the prognostic values of volume-based metabolic parameters for cN0 OTSCC.
In this study, we evaluated the prognostic values of volume-based metabolic PET parameters in patients with cN0 OTSCC in comparison to other clinical variables.
From January 2004 to December 2009, a total of 91 consecutive patients with pathologically proven SCC of the oral tongue who underwent 18F-FDG PET/CT for initial staging were identified by searching the institutional medical database. The subjects underwent X-ray computed tomography (CT) scan and/or magnetic resonance imaging (MRI) of the neck. Among these patients, a total of 57 patients with cN0 tongue cancer by CT, MRI of the neck, 18F-FDG PET/CT and physical exam were enrolled in this retrospective study. All patients were treated by resection of the primary tongue mass and elective neck dissection. The protocol of this retrospective study was reviewed and approved by the ethics committee of our institution.
All patients fasted for at least 6 hours prior to the PET/CT scan. Blood glucose level at the time of injection of 18F-FDG was less than 200 mg/dL in all patients. PET/CT was performed on two kinds of dedicated PET/CT scanners (Discovery LS or Discovery STe, GE Healthcare, Milwaukee, WI, USA). Among the 57 patients, 38 were analyzed by the Discovery LS PET/CT scanner and 19 were analyzed by the Discovery STe PET/CT scanner. No intravenous or oral contrast material was used.
In a Discovery LS scanner, whole-body CT was performed by a continuous spiral technique using an 8-slice helical CT (140 KeV, 40-120 mAs adjusted to the patients' body weight, a section width of 5 mm) 45 min after the injection of 18F-FDG (5.5 MBq/kg). After the CT scan, an emission scan was obtained from the thigh to the head for 4 minute per frame in 2D mode. Attenuation-corrected PET images (voxel size = 4.3 × 4.3 × 3.9 mm) using CT data were reconstructed using an ordered-subsets expectation maximization algorithm (28 subsets, two iterations). In the Discovery STe scanner, whole-body CT was performed by a continuous spiral technique using a 16-slice helical CT (140 KeV, 30-170 mAs with an AutomA mode, and a section width of 3.75 mm) 60 minute after the injection of 18F-FDG (5.5 MBq/kg). Following the CT scan, an emission scan was obtained from the thigh to the head for 2.5 minute per frame in 3D mode. Attenuation-corrected PET images (voxel size = 3.9 × 3.9 × 3.3 mm) using CT data were reconstructed using a 3D ordered-subsets expectation maximization algorithm (20 subsets, two iterations).
All 18F-FDG PET/CT images for initial staging were reviewed by experienced nuclear medicine physicians on a dedicated workstation (GE Advantage Workstation 4.4, GE Healthcare, Milwaukee, WI, USA) using volume viewer software, which provides an automatically delineated volume of interest (VOI) using an isocontour threshold method based on SUV (Fig. 1). MTV was defined as the total tumor volume segmented via a threshold SUV (18). Although many methods for the determination of an optimal threshold have been described (21-23), a standard method for clinical use has not been established. To identify a reliable representative method, several thresholds for determining the VOI boundary were adopted; mediastinal blood pool (MBP) activity (21) and fixed values of SUV at 2.5 (23), 3.0, 3.5 and 4.0. For the variable thresholds using MBP, a VOI consisting of 5 × 5 × 1 voxels was manually drawn at the aortic arch. The average SUV (SUVavg) plus two standard deviations of VOI was adopted as a threshold SUV for the tumor using MBP (TMBP). Using these five kinds of threshold SUVs, the VOIs of the primary tumor were automatically generated. The software calculated the SUVmax, SUVavg and MTV of the entire primary tumor according to the tumor VOIs. TLG was determined as a product of SUVavg and the number of voxels. If the primary tumor was not visualized or could not be distinguished from the background (n = 8), MTV was set as a single voxel with a volume of 0.1 cm3. In the case of SUVmax, an assumed default value of 2 was used for the convenience of statistical analysis. Those 2 values were lower than MTV or SUVmax of all visualized tumors.
Statistical analyses were performed using commercial software (IBM SPSS Statistics 19, IBM Inc., New York, NY, USA). Overall survival was defined as the time from the date of initial diagnosis to the date of death or final clinical follow-up. An event was defined as cancer or treatment-related death. The prognostic significances of SUVmax, MTV, TLG and other clinical variables were assessed by univariate and multivariate analyses using a Cox proportional hazards regression model. All tests were two-sided, and p values less than 0.05 were considered statistically significant. Data were expressed as the mean ± SD, if not otherwise mentioned.
Demographic and clinical characteristics of the patients are summarized in Table 1. Of 57 patients, 36 were male (63.2%), and the mean age was 49.9 ± 13.1 years with a range of 23-72 years. All patients had squamous cell carcinoma. The pathological tumor size was 1.9 ± 1.3 cm (range 0.3-6.0 cm). Elective neck dissection revealed that nine patients (15.9%) had cervical lymph node metastasis. According to the seventh edition of the American Joint Committee on Cancer (AJCC) staging system, 30 patients had stage I (52.6%), eight had stage II (14.0%), seven had stage III (12.3%), and the remaining 12 had stage IVA (21.1%) disease. Eight of 57 patients (14.0%) showed no demonstrable 18F-FDG uptake in the primary tongue lesion. Six of them had a lesion size less than 1.0 cm, and the remaining two had lesions measuring 1.6 and 3.0 cm, respectively. The mean SUVmax, SUVavg (cut-off SUV = 2.5), MTV (cut-off SUV = 2.5) and TLG (cut-off SUV = 2.5) of the primary lesions of all patients were 7.2 ± 5.5 (range, 2.0-30.0), 3.5 ± 1.6 (range, 1.0-7.3), 5.9 ± 8.2 cm3 (range, 0.1-35.0), and 30.8 ± 53.3 (range, 0.1-241.6), respectively. Among the thresholds used in this study, a cut-off of 2.5 showed the highest statistical significance in the prediction of overall survival. However, our conclusions regarding the prognostic values of metabolic parameters in predicting overall survival were not affected by the threshold method used. In other words, the results of the survival analysis using various cut-off SUVs were similar in terms of prognostic significance of volume-based PET parameters. Therefore, we described only the volume-based parameters with a cut-off of 2.5 for convenience.
The median follow-up duration was 45.0 months (range: 11.0-88.1 months). Of the 57 patients followed, 52 (91.2%) were alive at the final follow-up, and five patients (8.8%) had died. On a univariate analysis, SUVmax, SUVavg, MTV, TLG of primary tumor, pathological node (pN) stage, and AJCC stage were significant predictors of survival (Table 2). Among the five patients who died from the disease, four (80%) were AJCC stage IVA, and the remaining case (20%) was AJCC stage III. Three patients (60%) had cervical lymph node metastasis. According to the PET parameters, their mean SUVmax, SUVavg, MTV, and TLG were 14.0 ± 10.9, 5.2 ± 2.1, 15.4 ± 16.2 cm3, and 105.2 ± 116.7, respectively. On multivariate analyses (Table 3), MTV (hazard ratio [HR] = 2.698, 95% confidence interval [CI] = 1.083-6.721, p = 0.033), pN stage (HR = 10.888, 95% CI = 1.012-117.182, p = 0.049), and AJCC stage (HR = 13.220, 95% CI = 1.058-165.222, p = 0.045) were identified as significant prognostic factors associated with decreased overall survival. The 18F-FDG PET/CT images from a patient with squamous cell carcinoma of the tongue are shown in Figure 1. The SUVmax, SUVavg, MTV, and TLG of the primary mass were 30.3, 7.3, 29.9 cm3, and 218.3, respectively. The patient died of recurrent disease 11.0 months after cancer diagnosis.
Figure 2 demonstrates the Kaplan-Meier survival curve according to the MTV of the primary tumor. A cut-off MTV of 7.78 cm3 was determined by maximally selected log-rank statistics. The patients with high MTV showed significantly worse prognoses than those with low MTV (p = 0.037).
The present study may be the first investigation of volumetric parameters as prognostic factors specific to cN0 OTSCC. This study demonstrates that MTV, as a volumetric parameter of 18F-FDG PET, is an important independent prognostic factor for survival, in addition to cervical lymph node metastasis and AJCC stage.
Carcinoma of the tongue is known to have a high incidence of nodal metastasis, even during the early disease stage, and regional recurrence commonly causes treatment failure. In the present study, curative surgery revealed the presence of cervical lymph node metastasis in nine of 57 (15.9%) patients with cN0 OTSCC. Among them, three patients (33.3%) died due to disease recurrence. As expected, pN stage was a significant prognostic factor with a very high hazard ratio in both univariate and multivariate survival analyses (HR = 6.310 and 10.888, respectively). This study confirms that the presence of nodal metastasis in the neck is an important prognostic factor in cN0 OTSCC. AJCC stage was also a significant prognostic factor with a very high hazard ratio in both univariate and multivariate survival analyses (HR = 5.144 and 13.220, respectively).
In the current study, 18F-FDG PET/CT revealed that SUVmax, SUVavg, MTV, and TLG were significant prognostic factors in cN0 OTSCC. SUVmax, a semiquantitative index of tumor 18F-FDG uptake, has been shown to be a valuable parameter for the prognosis of survival in HNSCC. Several studies have indicated that a higher SUV of the primary tumor predicts a worse clinical outcome (15, 16, 24). However, in some recent studies, SUVmax of the primary tumor was not an independent prognostic factor for survival, exhibiting a poor predictive performance for treatment outcome (17, 25). In the present study, SUVmax was a significant prognostic factor for the univariate survival analysis; however, the parameter failed to be a significant prognostic factor on multivariate survival analysis. SUVmax is a single voxel value that may not represent total tumor uptake for the whole tumor mass and be vulnerable to statistical noise, which might explain the current results. On the contrary, volume-based parameters such as MTV and TLG could indicate total volume and total activity of the metabolically active tumor cells and might be less vulnerable to statistical variance. For HNSCC, the ability of volume-based parameters to predict outcome varies according to the tumor type and study method. It has been reported that MTV is an adverse prognostic factor for overall survival and is independent of other established prognostic factors (25). Our results also demonstrated that both MTV and TLG were significant prognostic factors on a univariate survival analysis. Furthermore, MTV of the primary tumor was an independent significant prognostic factor on multivariate survival analysis in cN0 OTSCC.
Metabolic tumor volume is obtained by 18F-FDG PET using dedicated software that measures the volume of 18F-FDG accumulation based on the high tumor-to-background intensity ratio following an attenuation correction (25). A study to compare CT, MRI, and 18F-FDG PET for the delineation of tumor volume in pharyngolaryngeal SCC with the results validated by the surgical specimen indicated that 18F-FDG PET is the most accurate modality for measuring tumor volume (26). Our previous study demonstrated that MTV was significantly correlated with occult metastasis with a very high hazard ratio (HR = 54.66) in patients with cN0 OTSCC (20). The present study added the prognostic value to MTV in cN0 OTSCC.
It is theoretically reasonable that TLG, which is a combination of SUV and MTV, represents both the degree of 18F-FDG uptake and the size of the metabolically active tumor, In addition, it is an ideal metabolic parameter of tumor burden. Several studies suggested that TLG was useful for the prognosis prediction and response to treatment evaluation in nasopharyngeal cancer, tonsilar carcinoma, esophageal cancer, etc (27-29). However, in this study, TLG failed to be a significant prognostic factor on multivariate survival analysis. These results might be associated with random variation and the relatively small number of subjects included in the multivariate survival analysis with many variables. Currently, it is not definite which PET parameter between MTV and TLG is more clinically useful in the oncology filed. Further studies using multicenter prospective large-sized design are warranted to clarify it.
Many oncologists would recommend adjuvant radiotherapy for large tumors if surgical margins are close to or involved with the tumor or after neck dissection involving many positive nodes. Conventional chemotherapy has a limited role in the primary management of carcinoma of the tongue but is sometimes considered as an adjunct in the treatment of advanced disease. In our study, the MTV of the primary tumor was an independent prognostic factor, irrespective of treatment modality. Our current findings on the volume-based parameter of PET suggest new ways to stratify cN0 OTSCC patients according to prognosis. With a more accurate prognosis, we can better select the patients with cN0 OTSCC who would benefit from adjuvant therapy and close surveillance after therapy.
The present study is limited by its retrospective nature and that the measurement of PET parameters was performed by two different PET scanner models using different protocols. Further, the diagnostic accuracy of cN0 could be argued in the present study. We combined all information from physical exams for palpable neck lesions, contrast-enhanced, dual-phase CT and/or MRI, and PET/CT to evaluate neck status in patients with OTSCC. The measurement of PET parameters was performed by two different PET scanner models with different protocols, which may have an influence on the SUV measurement. However, MTV was consistently identified as a significant prognostic predictor, irrespective of the threshold determination methods for tumor boundary, which suggests that the effect of scanner model and protocol might not significantly affect our results. To clarify, additional studies with the same scanner are needed before it will be generally accepted. The number of patients in the present study population was relatively small for performing multivariate analyses. Finally, 22.8% of primary tumors (13/57) had a lesion size with a maximum diameter less than 1 cm, which might underestimate volumetric PET parameters due to partial volume effect. We cannot exclude the possibility that this might have an effect on the results of the survival analysis. Therefore, our study involves generating hypotheses to support the clinical value of volumetric functional assessment in patients with OTSCC. Further prospective multicenter validation studies are needed with a larger number of patients and standardized imaging protocols across different PET/CT scanners.
In conclusion, the present study suggests that MTV, as a volumetric parameter of 18F-FDG PET, is an independent prognostic factor for survival in addition to pN stage and AJCC stage in cN0 OTSCC patients. Therefore, 18F-FDG PET may be helpful for selecting candidates for adjuvant therapy and close follow-up after therapy in patients with cN0 OTSCC. Although there is insufficient standardization and inadequate validation for the implementation of the volumetric functional assessment in clinical practice, we present our results as a hypothesis-generating study that warrants further confirmation. Additional multicenter prospective studies with larger patient cohorts are needed to validate the prognostic utility of this promising functional biomarker derived from 18F-FDG PET/CT.
Figures and Tables
Fig. 1
18F-FDG PET/CT images from 35-year-old male patient with squamous cell carcinoma of tongue.
Primary tumor uptake is well visualized on MIP image (A). Segmented VOIs are shown on transverse (B), sagittal (C), coronal (D), and fused PET/CT (E) images. MIP = maximum-intensity-projection, VOI = volume of interest. 18F-FDG PET/CT = 18F-fluorodeoxyglucose positron emission tomography/CT
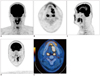
Fig. 2
Kaplan-Meier survival curve according to metabolic tumor volume of primary tumor in clinically node-negative oral tongue squamous cell carcinoma.

Table 2
Univariate Analysis for Overall Survival Using Cox Proportional-Hazard Model

Note.- *Significant. HR = hazard ratio, CI = confidence interval, AJCC = American Joint Committee on Cancer, SUVmax = maximum standardized uptake value, SUVavg = average standardized uptake value, MTV = metabolic tumor volume, TLG = total lesion glycolysis, pN = pathological N, pT = pathological T, adj Tx = adjuvant therapy
References
1. Bello IO, Soini Y, Salo T. Prognostic evaluation of oral tongue cancer: means, markers and perspectives (I). Oral Oncol. 2010. 46:630–635.
2. Bello IO, Soini Y, Salo T. Prognostic evaluation of oral tongue cancer: means, markers and perspectives (II). Oral Oncol. 2010. 46:636–643.
3. Regezi JA, Sciubba JJ, Jordan RCK. Oral pathology: Clinical, pathologic correlations. 2007. 5th ed. St. Louis, MO: Saunders Elsevier.
4. Leipzig B, Cummings CW, Chung CT, Johnson JT, Sagerman RH. Carcinoma of the anterior tongue. Ann Otol Rhinol Laryngol. 1982. 91(1 Pt 1):94–97.
5. Rusthoven K, Ballonoff A, Raben D, Chen C. Poor prognosis in patients with stage I and II oral tongue squamous cell carcinoma. Cancer. 2008. 112:345–351.
6. Sathyan KM, Sailasree R, Jayasurya R, Lakshminarayanan K, Abraham T, Nalinakumari KR, et al. Carcinoma of tongue and the buccal mucosa represent different biological subentities of the oral carcinoma. J Cancer Res Clin Oncol. 2006. 132:601–609.
7. Zanation AM, Sutton DK, Couch ME, Weissler MC, Shockley WW, Shores CG. Use, accuracy, and implications for patient management of [18F]-2-fluorodeoxyglucose-positron emission/computerized tomography for head and neck tumors. Laryngoscope. 2005. 115:1186–1190.
8. Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008. 49:480–508.
9. Shin KM, Lee KS, Shim YM, Kim J, Kim BT, Kwon OJ, et al. FDG PET/CT and mediastinal nodal metastasis detection in stage T1 non-small cell lung cancer: prognostic implications. Korean J Radiol. 2008. 9:481–489.
10. Baek CH, Chung MK, Jeong HS, Son YI, Choi J, Kim YD, et al. The clinical usefulness of (18)F-FDG PET/CT for the evaluation of lymph node metastasis in periorbital malignancies. Korean J Radiol. 2009. 10:1–7.
11. Adams S, Baum RP, Stuckensen T, Bitter K, Hör G. Prospective comparison of 18F-FDG PET with conventional imaging modalities (CT, MRI, US) in lymph node staging of head and neck cancer. Eur J Nucl Med. 1998. 25:1255–1260.
12. Ng SH, Yen TC, Liao CT, Chang JT, Chan SC, Ko SF, et al. 18F-FDG PET and CT/MRI in oral cavity squamous cell carcinoma: a prospective study of 124 patients with histologic correlation. J Nucl Med. 2005. 46:1136–1143.
13. Byers RM, El-Naggar AK, Lee YY, Rao B, Fornage B, Terry NH, et al. Can we detect or predict the presence of occult nodal metastases in patients with squamous carcinoma of the oral tongue? Head Neck. 1998. 20:138–144.
14. Werning JW, Heard D, Pagano C, Khuder S. Elective management of the clinically negative neck by otolaryngologists in patients with oral tongue cancer. Arch Otolaryngol Head Neck Surg. 2003. 129:83–88.
15. Minn H, Lapela M, Klemi PJ, Grénman R, Leskinen S, Lindholm P, et al. Prediction of survival with fluorine-18-fluoro-deoxyglucose and PET in head and neck cancer. J Nucl Med. 1997. 38:1907–1911.
16. Allal AS, Slosman DO, Kebdani T, Allaoua M, Lehmann W, Dulguerov P. Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-[18F] fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys. 2004. 59:1295–1300.
17. Choi KH, Yoo IR, Han EJ, Kim YS, Kim GW, Na SJ, et al. Prognostic value of metabolic tumor volume measured by 18F-FDG PET/CT in locally advanced head and neck squamous cell carcinomas treated by surgery. Nucl Med Mol Imaging. 2011. 45:43–51.
18. Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol. 2010. 17:115–122.
19. Kim BS, Kim IJ, Kim SJ, Nam HY, Pak KJ, Kim K, et al. The prognostic value of the metabolic tumor volume in FIGO stage IA to IIB cervical cancer for tumor recurrence: Measured by F-18 FDG PET/CT. Nucl Med Mol Imaging. 2011. 45:36–42.
20. Chung MK, Jeong HS, Son YI, So YK, Park GY, Choi JY, et al. Metabolic tumor volumes by [18F]-fluorodeoxyglucose PET/CT correlate with occult metastasis in oral squamous cell carcinoma of the tongue. Ann Surg Oncol. 2009. 16:3111–3117.
21. Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007. 25:571–578.
22. Lee P, Weerasuriya DK, Lavori PW, Quon A, Hara W, Maxim PG, et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiat Oncol Biol Phys. 2007. 69:328–333.
23. Nestle U, Kremp S, Schaefer-Schuler A, Sebastian-Welsch C, Hellwig D, Rübe C, et al. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-small cell lung cancer. J Nucl Med. 2005. 46:1342–1348.
24. Roh JL, Pae KH, Choi SH, Kim JS, Lee S, Kim SB, et al. 2-[18F]-Fluoro-2-deoxy-D-glucose positron emission tomography as guidance for primary treatment in patients with advanced-stage resectable squamous cell carcinoma of the larynx and hypopharynx. Eur J Surg Oncol. 2007. 33:790–795.
25. La TH, Filion EJ, Turnbull BB, Chu JN, Lee P, Nguyen K, et al. Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009. 74:1335–1341.
26. Daisne JF, Duprez T, Weynand B, Lonneux M, Hamoir M, Reychler H, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology. 2004. 233:93–100.
27. Chan SC, Chang JT, Lin CY, Ng SH, Wang HM, Liao CT, et al. Clinical utility of 18F-FDG PET parameters in patients with advanced nasopharyngeal carcinoma: predictive role for different survival endpoints and impact on prognostic stratification. Nucl Med Commun. 2011. 32:989–996.
28. Moon SH, Choi JY, Lee HJ, Son YI, Baek CH, Ahn YC, et al. Prognostic value of (18) F-FDG PET/CT in patients with squamous cell carcinoma of the tonsil: Comparisons of volume-based metabolic parameters. Head Neck. 2012. doi:10.1002/hed.22904 [Epub ahead of print].
29. Hatt M, Visvikis D, Pradier O, Cheze-le Rest C. Baseline 18F-FDG PET image-derived parameters for therapy response prediction in oesophageal cancer. Eur J Nucl Med Mol Imaging. 2011. 38:1595–1606.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download