Abstract
Eccrine spiradenomas are rare, benign, cutaneous tumors that originate in the sweat glands. Eccrine spiradenomas in the breast are very rare and only a few cases have been reported. We report here on the case of a 47-year-old woman with superficial masses in the breast and these masses had gradually increased in size during follow-up. They were confirmed to be an eccrine spiradenoma on pathologic examination. There have been a few reports about the radiologic findings of eccrine spiradenomas of the breast. This is the first case of an eccrine spiradenoma in the breast that was characterized by multiple imaging modalities, including mammography, ultrasonography and MRI. The lesion in our patient was first diagnosed as an epidermal inclusion cyst based on the imaging findings and the mass's superficial location. Although the mammographic and ultrasonographic imaging findings of eccrine spiradenomas and epidermal inclusion cysts are similar, the MRI findings are different between epidermal inclusion cysts and eccrine spiradenomas. Eccrine spiradenomas should be considered in the differential diagnosis of cutaneous and subcutaneous lesions of the breast.
Eccrine spiradenomas were first reported as rare, benign, adnexal tumors of the skin in 1956 (1). Eccrine spiradenomas typically present as painful, slow-growing, solitary masses on the head or upper trunk, and they usually occur during the fourth or fifth decade of life, and without a predilection for either gender (2). Eccrine spiradenomas in the breast are very rare (2) and little is known about the corresponding radiologic findings. We report here on the case of an eccrine spiradenoma in the breast of a 47-year-old woman and we present the mammography, ultrasound (US) and breast MRI findings.
A 47-year-old woman sought evaluation at our hospital for a palpable and occasionally painful lump that had been gradually increasing in size and it had persisted for three years in the left breast. The physical examination revealed a tender, movable, palpable mass in the left upper breast. Mammography demonstrated a well-defined, isodense mass in the left upper center quadrant (Fig. 1A, B). A breast US exam showed three well-defined hypoechoic masses in the subcutaneous layer. The lesions had first been noticed three years previously, and at that time the lesions were thought to be benign masses such as epidermal inclusion cysts on breast US due to the imaging findings and their superficial location in the skin and subcutaneous layer (Fig. 1C). It was recommended that the patient undergo routine follow-up. The current US examination demonstrated an apparent increase in the size of the masses as compared to that seen on the breast US examination performed three years earlier (Fig. 1D), and the lesions were shown to be solid rather than cystic. The patient underwent an US-guided 14-gauge core needle biopsy for pathologic evaluation. The pathologic findings on core needle biopsy were consistent with an eccrine spiradenoma of the breast. A breast MRI exam using a dedicated bilateral breast coil showed low signal intensity on the T1-weighted images, low- to intermediate-signal intensity on the T2-wighted turbo spin echo images, high signal intensity on the turbo inversion recovery magnitude (TIRM) sequence and homogeneous enhancement on the gadolinium-enhanced T1-weighted images (Fig. 1E-H). The patient then underwent surgical excision for pain relief and due to the potential for malignancy. The surgical specimen showed superficially located masses in the breast that each measured approximately 1 cm in size (Fig. 1I). Microscopy demonstrated the presence of masses in the dermis without obvious continuity with the epidermis (Fig. 1J). A histologic examination showed a characteristic biphasic population of outer small cells with darkly staining nuclei surrounding larger cells with pale cytoplasm and tightly packed cells arranged in a jigsaw puzzle pattern without epidermal connections (Fig. 1K). A histopathologic examination confirmed the diagnosis of a benign, adnexal skin tumor in the breast, and this was all consistent with an eccrine spiradenoma.
Eccrine spiradenomas are benign tumors of the sweat glands and they are characteristically located in the superficial and deep dermis. Eccrine spiradenomas have no gender predilection, they usually occur on the trunk or extremities, they are 1-2 cm in size and they are occasionally associated with pain and tenderness (3). Eccrine glands are present throughout the skin, but they are most frequent on the palms, soles and axillae. The majority of eccrine spiradenomas involve the trunk and extremities; lesions involving the breast are very rare (4). Of note, malignant changes and systemic metastasis of eccrine spiradenoma of the breast have been reported (2, 3). Malignant changes of an eccrine spiradenoma are extremely rare (2) and they generally arise from long-standing benign eccrine spiradenomas. To diagnose an eccrine spiradenoma, a biopsy should be obtained for pathologic evaluation (5). An eccrine spiradenoma is a highly cellular, cutaneous and subcutaneous tissue tumor with clear margins and it is composed of two types of cells (small, dark-staining basaloid cells and larger pale-staining cells). At low power magnification, a spiradenoma appears as a solid neoplasm composed of a single mass or a few masses of basaliod cells. At higher magnification, two distinct populations of neoplastic epithelial cells can be seen as dark and pale cells (6).
A few classic cases of eccrine spiradenomas have been reported (3, 6). However, the imaging findings of eccrine spiradenomas of the breast have not been clearly demonstrated because of the rare incidence of eccrine spiradenomas and the lack of imaging studies. In 2008, Jin et al. (7) reported the US findings of an eccrine spiradenoma in the upper arm that revealed a well-defined lobulating mass with heterogeneous hypoechogenicity in the deep portion of the dermis and superficial subcutaneous fat layer without connections to the epidermis, and there was no extension into the muscular structures. The imaging findings of our case corresponded well with the imaging findings reported by Jin et al. (7). The US findings of the current case were characterized as well-circumscribed, oval-shaped, hypoechoic masses in the cutaneous and subcutaneous layer, and all the masses were surrounded by echogenic lines that represent the dermal layer, which are findings suggestive of a cutaneous origin. The lesion in our patient was first diagnosed as an epidermal inclusion cyst based on the images and the masses' superficial location. Epidermal inclusion cysts are well-defined, round- or oval-shaped, iso- or hyper-dense masses on mammography; the US findings also include round- or oval-shaped, well-demarcated masses with variable echogenicity (hypo- or hyper-echogenicity) due to variable amounts of keratin debris, and epidermal inclusion cysts are superficially located in the cutaneous or subcutaneous layers. Posterior acoustic enhancements are common, but epidermal inclusion cysts frequently mimic solid masses due to their variable echogenicity. It is difficult to differentiate eccrine spiradenomas from epidermal inclusion cysts based solely on mammographic and US imaging studies due to their similar appearance on imaging and their superficial location. Usually, epidermal inclusion cysts are not recommended for fine needle aspiration or a core needle biopsy because the contents, which can leak out after needle biopsy, often irritate the surrounding tissues and they evokes an inflammatory response, and an abscess may also occur; however, a biopsy is needed to pathologically diagnose an eccrine spiradenoma.
Only one study (6) has reported the MRI appearance of multiple eccrine spiradenomas that were distributed in the trunk, face and extremities. Cystic or solid masses with low signal intensity on the T1-weighted images and high signal intensity on the short T1 inversion recovery images were seen. Thus far, there have been no MRI reports of eccrine spiradenomas in the breast. In the case presented herein, the MR images showed low signal intensity on the T1-weighted images, high signal intensity on the TIRM images and intermediate- to low-signal intensity on the T2-weighted images with homogeneous enhancement on the T1-weighted enhanced images. In the differential diagnosis of epidermal inclusion cysts, Hong et al. (8) reported that subcutaneous epidermal inclusion cysts in the trunk and extremities showed slightly high signal intensity on the T1-weighted images with some bright foci, a high signal intensity background with variable low signal components on the T2-weighted images and peripheral thin rim enhancement on the fat-suppressed T1-weighted enhanced images; the study by Hong et al. (8) is the largest series of MR images of pathologically-confirmed epidermal inclusion cysts. The signal intensities of epidermal inclusion cysts might be the result of the cystic content filled with keratin materials and bounded by a wall of stratified squamous epithelium. An epidermal inclusion cyst is more likely than another subcutaneous lesion when the T2-weighted image shows variable low signal components within the lesion. According to Hong et al. (8) and the current case, the MRI findings of epidermal inclusion cysts and eccrine spiradenomas are different and so these findings might be used in making the differential diagnosis.
In summary, we have described a rare case of a sweat gland tumor in the breast, an eccrine spiradenoma, with the use of imaging modalities, including mammography, US and MRI. Although an eccrine spiradenoma may resemble an epidermal inclusion cyst on mammography and US, there is a difference between the MRI findings of epidermal inclusion cysts and eccrine spiradenomas.
Figures and Tables
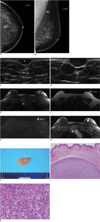
Fig. 1
Eccrine spiradenoma arising in breast in 47-year-old woman.
A, B. Routine mammography was performed and this showed two well-defined nodular densities in left upper central quadrant. C, D. Breast US was performed on first visit three years previously (C). US demonstrated presence of three well-defined, oval-shaped, homogenously hypoechoic masses within skin layer (white stars), with sizes of 9 × 4 mm, 8 × 5 mm and 4 × 2 mm, and mild posterior acoustic enhancement. As compared to previous US, current breast US (D) shows presence of masses that have apparently increased in size in skin and subcutaneous layer. Maximum diameter of masses had increased from 9 to 17 mm and from 8 to 9 mm. More importantly, three masses are surrounded by echogenic lines (white arrows) that represent dermal layer. This appearance suggests cutaneous lesion. E-H. Breast MRI demonstrates well-defined masses in left breast with low signal intensity on axial T1-weighted images (repetition time [TR]/echo time [TE]: 4.6/1.7) (E), intermediate- to low-signal intensity on T2-weighted turbo spin echo (TSE) images (TR/TE: 3800/90) (F), high signal intensity on turbo inversion recovery magnitude image (TIRM, TR/TE/inversion time [TI]: 5620/88/230) (G), and fat-suppressed gadolinium-enhanced T1-wighted image shows masses with homogeneous enhancement and linear structure that is biopsy needle track from skin to masses (H). I. Surgical specimen is 3.8 × 3.0 × 2.0 cm skin tissue specimen attached to breast tissue. There are two well-defined, yellow-white solid masses, 1.4 × 1.0 × 0.8 cm and 1.0 × 1.0 × 0.8 cm in size (arrows). J, K. Photomicrograph of specimen (Hematoxylin & Eosin staining) demonstrates eccrine spiradenoma composed of characteristic double population of outer small cells with darkly staining nuclei surrounding larger cells with pale cytoplasm and tightly packed cells arranged in jigsaw puzzle pattern without epidermal connections (original magnification, × 100 & × 400, respectively).
References
1. Kersting DW, Helwig EB. Eccrine spiradenoma. AMA Arch Derm. 1956. 73:199–227.
2. Ribeiro-Silva A, Shaletich C, Careta RS, Kazava DK, Siqueira MC, Ponton F. Spiradenocarcinoma of the breast arising in a long-standing spiradenoma. Ann Diagn Pathol. 2004. 8:162–166.
3. Leonard N, Smith D, McNamara P. Low-grade malignant eccrine spiradenoma with systemic metastases. Am J Dermatopathol. 2003. 25:253–255.
4. Bosch MM, Boon ME. Fine-needle cytology of an eccrine spiradenoma of the breast: diagnosis made by a holistic approach. Diagn Cytopathol. 1992. 8:366–368.
5. Han YD, Huan Y, Deng JL, Zhang YG, Zhang CH. MRI appearance of multiple eccrine spiradenoma. Br J Radiol. 2007. 80:E27–E29.
6. Dijkhuizen T, van den Berg E, Nikkels PG, Hoekstra HJ, de Jong B. Cytogenetics of a case of eccrine spiradenoma. Hum Pathol. 1992. 23:1085–1087.
7. Jin W, Kim GY, Lew BL, Yang DM, Kim HC, Ryu JK, et al. Sonographic findings of an eccrine spiradenoma: case report and literature review. J Ultrasound Med. 2008. 27:813–818.
8. Hong SH, Chung HW, Choi JY, Koh YH, Choi JA, Kang HS. MRI findings of subcutaneous epidermal cysts: emphasis on the presence of rupture. AJR Am J Roentgenol. 2006. 186:961–966.
9. Chen JT, Dahmash NS, Ravin CE, Heaston DK, Putman CE, Seigler HF, et al. Metastatic melanoma in the thorax: report of 130 patients. AJR Am J Roentgenol. 1981. 137:293–298.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download