1. Janz D, Durner M. Engel J, Pedley TA, editors. Juvenile myoclonic epilepsy. Epilepsy: A Comprehensive Textbook. 1998. Philadelphia: Lippincott-Raven;2389–2400.
2. Panayiotopoulos CP, Obeid T. Juvenile myoclonic epilepsy: an autosomal recessive disease. Ann Neurol. 1989. 25:440–443.
3. Liu AW, Delgado-Escueta AV, Serratosa JM, Alonso ME, Medina MT, Gee MN, et al. Juvenile myoclonic epilepsy locus in chromosome 6p21.2-p11: linkage to convulsions and electroencephalography trait. Am J Hum Genet. 1995. 57:368–381.
4. Durner M, Sander T, Greenberg DA, Johnson K, Beck-Mannagetta G, Janz D. Localization of idiopathic generalized epilepsy on chromosome 6p in families of juvenile myoclonic epilepsy patients. Neurology. 1991. 41:1651–1655.
5. Elmslie FV, Rees M, Williamson MP, Kerr M, Kjeldsen MJ, Pang KA, et al. Genetic mapping of a major susceptibility locus for juvenile myoclonic epilepsy on chromosome 15q. Hum Mol Genet. 1997. 6:1329–1334.
6. Santiago-Rodriguez E, Harmony T, Fernandez-Bouzas A, Hernandez-Balderas A, Martinez-Lopez M, Graef A, et al. Source analysis of polyspike and wave complexes in juvenile myoclonic epilepsy. Seizure. 2002. 11:320–324.
7. Savic I, Lekvall A, Greitz D, Helms G. MR spectroscopy shows reduced frontal lobe concentration of N-acetyl aspartate in patients with juvenile myoclonic epilepsy. Epilepsia. 2000. 41:290–296.
8. Devinsky O, Gershengorn J, Brown E, Perrine K, Vazquez B, Luciano D. Frontal function in juvenile myoclonic epilepsy. Neuropsychiatry Neuropsychol Behav Neurol. 1997. 10:243–246.
9. Koepp MJ, Richardson MP, Brooks DJ, Cunningham VJ, Duncan JS. Central benzodiazepine/gamma-aminobutyric acid A receptors in idiopathic generalized epilepsy: an [11C]-flumazenil positron emission tomography study. Epilepsia. 1997. 38:1089–1097.
10. Swartz BE, Simpkins F, Halgren E, Mandelkern M, Brown C, Krisdakumtorn T, et al. Visual working memory in primary generalized epilepsy: an 18FDG-PET study. Neurology. 1996. 47:1203–1212.
11. Gelisse P, Genton P, Samuelian JC, Thomas P, Bureau M. Psychiatric disorders in juvenile myoclonic epilepsy. Rev Neurol. 2001. 157:297–302.
12. Gelisse P, Genton P, Raybaud C, Thomas P, Dravet C. Structural brain lesions do not influence the prognosis of juvenile myoclonic epilepsy. Acta Neurol Scand. 2000. 102:188–191.
13. Woermann FG, Sisodiya SM, Free SL, Duncan JS. Quantitative MRI in patients with idiopathic generalized epilepsy. Evidence of widespread cerebral structural changes. Brain. 1998. 121:1661–1667.
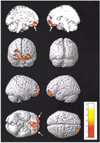
14. Woermann FG, Free SL, Koepp MJ, Sisodiya SM, Duncan JS. Abnormal cerebral structure in juvenile myoclonic epilepsy demonstrated with voxel-based analysis of MRI. Brain. 1999. 122:2101–2108.
15. Woermann FG, Free SL, Koepp MJ, Ashburner J, Duncan JS. Voxel-by-voxel comparison of automatically segmented cerebral gray matter-A rater-independent comparison of structural MRI in patients with epilepsy. Neuroimage. 1999. 10:373–384.
16. Jack CR Jr, Bentley MD, Twomey CK, Zinsmeister AR. MR imaging-based volume measurements of the hippocampal formation and anterior temporal lobe: validation studies. Radiology. 1990. 176:205–209.
17. Johannessen CU. Mechanisms of action of valproate: a commentatory. Neurochem Int. 2000. 37:103–110.
18. Chapman AG, Riley K, Evans MC, Meldrum BS. Acute effects of sodium valproate and gamma-vinyl GABA on regional amino acid metabolism in the rat brain: incorporation of 2-[14C]glucose into amino acids. Neurochem Res. 1982. 7:1089–1105.
19. DeLong MR. Kandal ER, Schwartz JH, Jessell TM, editors. The basal ganglia. Principles of neural science. 2000. 4th ed. New York: McGraw-Hill;853–867.
20. Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989. 30:389–399.
21. Witelson FS. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989. 112:799–835.
22. Duvernoy HM. The human brain: surface, three-dimensional sectional anatomy with MRI, and blood supply. 1991. New York: Springer-Verlag.
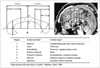
23. Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997. 120:141–157.
24. Aylward EH, Anderson NB, Bylsma FW, Wagster MV, Barta PE, Sherr M, et al. Frontal lobe volume in patients with Huntington's disease. Neurology. 1998. 50:252–258.
25. Arndt S, Swayze V, Cizadlo T, O'Leary D, Cohen G, Yuh WT, et al. Evaluating and validating two methods for estimating brain structure volumes: tessellation and simple pixel counting. Neuroimage. 1994. 1:191–198.
26. Arndt S, Cohen G, Alliger RJ, Swayze VW 2nd, Andreasen NC. Problems with ratio and proportion measures of imaged cerebral structures. Psychiatry Res. 1991. 40:79–89.
27. Pujol J, Vendrell P, Junque C, Marti-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann Neurol. 1993. 34:71–75.
28. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001. 14:21–36.
29. Moses P, Courchesne E, Stiles J, Trauner D, Egaas B, Edwards E. Regional size reduction in the human corpus callosum following pre- and perinatal brain injury. Cereb Cortex. 2000. 10:1200–1210.
30. Pelletier J, Suchet L, Witjas T, Habib M, Guttmann CR, Salamon G, et al. A longitudinal study of callosal atrophy and interhemispheric dysfunction in relapsing-remitting multiple sclerosis. Arch Neurol. 2001. 58:105–111.
31. Hampel H, Teipel SJ, Alexander GE, Horwitz B, Teichberg D, Schapiro MB, et al. Corpus callosum atrophy is a possible indicator of region- and cell type-specific neuronal degeneration in Alzheimer disease: a magnetic resonance imaging analysis. Arch Neurol. 1998. 55:193–198.
32. Teipel SJ, Hampel H, Pietrini P, Alexander GE, Horwitz B, Daley E, et al. Region-specific corpus callosum atrophy correlates with the regional pattern of cortical glucose metabolism in Alzheimer disease. Arch Neurol. 1999. 56:467–473.
33. Meschaks A, Lindstrom P, Halldin C, Farde L, Savic I. Regional reductions in serotonin 1A receptor binding in juvenile myoclonic epilepsy. Arch Neurol. 2005. 62:946–950.
34. Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, et al. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004. 161:99–108.
35. Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005. 26:1271–1274.
36. Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, et al. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002. 17:657–669.







 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download