Abstract
Objective
This study assessed the outcomes of using vascular closure devices following percutaneous transfemoral endovascular procedures in the patients who were treated with heparin, abciximab or thrombolytics (urokinase or t-PA) during the procedures.
Materials and Methods
From March 28, 2003 to August 31, 2004, we conducted a prospective and randomized study in which 1,676 cases of 1,180 patients were treated with one of the two different closure devices (the collagen plug device was Angio-Seal™; the suture-mediated closure device was The Closer S™) at the femoral access site after instituting percutaneous endovascular procedures. Among the 1,676 cases, 108 cases (the drug group) were treated with heparin only (n = 94), thrombolytics only (n = 10), heparin and thrombolytics (n = 3), or abciximab and thrombolytics (n = 1) during the procedures; 1,568 cases (the no-drug group) were treated without any medication. We compared the efficacy and complications between the two groups. Of the drug group, 42 cases underwent arterial closures with the collagen plug devices and 66 cases underwent arterial closures with the suture-mediated closure devices. We also compared the efficacy and complications between these two groups.
Results
The immediate hemostasis rates were 92.9% (1,456/1,568) in the no-drug group and 91.7% (99/108) in the drug group. Early complications occurred in four cases of the drug group. These included two episodes of rebleeding with using the Closer S, which required manual compression for at least 10 minutes, and two episodes of minor oozing with using one Angio-Seal and one Closer S, which required two hours of additional bed rest. There was no late complication. So, the total success rates were 90.8% (1,423/1,568) in the no-drug group and 88.0% (95/108) in the drug group. These results were not significantly different between the two groups (p = 0.34). In the drug group, the difference of the successful hemostasis rate between the collagen plug devices and the suture-mediated devices was also not statistically significant (92.9% vs. 84.8%, respectively; p = 0.21).
The femoral artery is the most frequent access site that is targeted for percutaneous endovascular procedures. Arterial puncture sites after catheterization tend to be associated with bleeding, hematoma, pseudoaneurysm and a variety of other complications. These complications may also be associated with significant patient discomfort, an increased hospital stay, blood transfusion and sometimes vascular surgery (1). Up to 10% of these patients complain of significant local complications, including hematoma and pseudoaneurysms. In 1-2% of such cases, these complications require vascular surgical intervention or transfusion (2-4). The rates of these complications may depend on the operator's experience, the type of intervention attempted, the introducer size and primarily, on the duration of the manual compression (5).
Therefore, several closure devices have been developed to facilitate hemostasis, allow early ambulation and discharge, and to ameliorate patient discomfort (1). One of these devices utilizes collagen for the management of access site closure (VasoSeal, Angio-Seal, Duett). Alternatively, a percutaneous suturing device (Prostar, Techstar, Closer) allows for surgical closure of the femoral artery with very little trauma being incurred by the overlying tissue (4, 6, 7). In some reports, the suture-based closure device has been demonstrated to be both safe and effective for the induction of immediate hemostasis and early ambulation, and there was no concomitant increase in the risk of bleeding complications (8, 9). Koreny et al. (10) suggested that the arterial puncture closing devices appeared to be effective in terms of reducing the time that is necessary for hemostasis, but complications such as hematoma and pseudoaneurysm formation occurred more often than for standard manual compression, as based on the meta-analysis of 30 randomized trials.
Anyway, the vascular closure devices have been frequently used for the patients who have recently undergone peripheral vascular diagnostic and therapeutic procedures (11, 12). These devices are particularly used for patients who have either been heparinized or heavily anticoagulated (13). An increase in the number of available diagnostic and therapeutic interventional procedures, as well as the use of new antiplatelet agents during angioplasty, has coincided with an upswing in the frequency with which these devices are used (11). These devices share common goals of achieving prompt hemostasis, even in a setting of systemic anticoagulation, allowing for earlier ambulation and helping to prevent groin complications (14).
In this study, we assessed the safety and efficacy of using two closure devices, the collagen plug device (Angio-Seal™; Daig Corporation, Minnetonka, MN) and the suture-mediated device (The Closer S™; Abbott Corporation, Redwood City, CA), after performing percutaneous endovascular procedures in patients who received heparin, abciximab or thrombolytics (urokinase or t-PA) during the procedures. We also compared the outcomes of the two vascular closure devices.
The choice of methods that were used to obtain hemostasis, including manual compression or vascular closure devices, was left to the discretion of patients after they were given an explanation about the efficacy and side effects of the vascular closure devices. The use of the vascular closure devices was attempted in all patients if they gave their written informed consents prior to the procedures. The exclusion criteria for the study included difficulty in puncturing the artery, severe peripheral vascular disease, marked obesity, an age < 15 years and an arterial sheath < 4 Fr or > 8 Fr.
From March 28, 2003 to August 31, 2004, 1,180 patients who underwent 1,676 percutaneous transfemoral endovascular procedures at the Samsung Medical Center (SMC) were eligible for our study. These patients were randomized into two groups that received either the collagen plug device (Angio-Seal™) or the suture-mediated device (the Closer S™). Of our 1,676 cases, 961 cases were treated with Angio-Seal and 715 cases were treated with the Closer S. The interventional radiologists and neuroradiologists at SMC had experience with 322 cases of Angio-Seal and 97 cases of Closer S at the beginning of this study.
The placement of either type of closure device was performed using the manufacturer's recommended technique. Ambulation was normally initiated two hours after the placement of the relevant arterial closure device. The demographic and clinical outcome data were prospectively collected using a standardized "procedural data sheet" and the data was recorded on the day on which the procedure was performed, 24 hours afterward and at one month, or at the time when the complications were noted. The procedural data included the type of intervention, the procedure data, the sheath size, the procedure-related drug dose and the number of previous punctures. Major or minor complications, as well as the time of events, were also recorded. In all instances, further anticoagulation or use of antiplatelet agents was decided upon by a consensus of the interventional radiologists and the attending physicians.
In 108 cases (100 patients) of the 1,676 cases, heparin, thrombolytics or abciximab was administered intra-arterially or intravenously during the procedures. These 108 cases (the drug group) were then divided into the two groups based on the type of closure devices used and the drugs that were administered. We analyzed the immediate successful hemostasis, complications and the total success rates of these groups, and these cases were then compared with the other 1,568 cases (the no-drug group) in which no anticoagulant, thrombolytic or antiplatelet drug was used.
The complications were categorized as early (within 24 hours) or late (within 1 month). The minor complications included any rebleeding or oozing from the puncture site that proved to be controllable by manual compression, as well as infections that were treatable with oral antibiotics. The major complications were also assessed. These included the need for vascular surgery, hemorrhage requiring transfusion, a pseudoaneurysm or arteriovenous fistula, arterial occlusion or distal arterial embolism, and infections that were treatable only by the administration of IV antibiotics or debridement.
Immediate hemostasis was defined as achieving hemostasis after the deployment of the device, with or without applying three minutes or less of manual compression. Total success was defined as immediate hemostasis with no complications.
The data are expressed as means value±standard deviation, unless otherwise indicated. Comparative analyses were carried out with using standard chi-square tests between each of the closure device groups, as well as between the patient groups based on the drugs that were administered. Fischer's exact test was used if the expected cell count for a 2×2 table was < 5. The two-tailed unpaired Student t test was used to analyze continuous variables. These comparisons were performed using the SPSS version 11.0 software package (SPSS Inc., Chicago, IL), and a p value of ≤0.05 was considered to be significant.
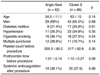
Of the 1,676 cases in the baseline study, 961 cases were closed with using the Angio-Seal and 715 cases were closed with using the Closer S. Table 1 displays the baseline outcomes of the two groups, according to the vascular closure devices that were used. Immediate hemostasis was achieved in 95.2% of the cases treated with the Angio-Seal and in 89.5% of the cases treated with the Closer S (p < 0.001). Total success was achieved in 92.6% of the cases with using of the Angio-Seal and in 87.8% of the cases with using the Closer S (p < 0.01). There were no statistically significant differences between the two groups with regard to early or late complications.
Of the 1,676 cases, 108 cases (100 patients) were treated with heparin only (n = 94), thrombolytics only (n = 10), combined heparin and thrombolytics (n = 3), or combined abciximab and thrombolytics (n = 1) during the procedures. The mean dose of heparin was 4,238±1,287.6 units, the mean dose of urokinase was 241,667±66,458 units, the mean dose of intravenous and/or intraarterial tissue plasminogen activator (t-PA) was 32.3±16.6 mg and the mean dose of abciximab was 5 mg.
These 108 cases underwent a variety of procedures including 10 (9.3%) diagnostic transfemoral carotid angiographies (TFCA) and 98 (90.7%) interventional procedures. The interventional procedures included 51 (47.2%) embolizations of intracranial aneurysms, 15 (13.9%) intra-arterial thrombolysis procedures for stroke patients, 15 (13.9%) intracranial stent placements, eight (7.4%) iliac stent placements, three (2.8%) intracranial angioplasties, two (1.9%) balloon occlusions of giant carotid aneurysms, two (1.9%) peripheral subintimal angioplasties, one (0.9%) embolization of a dural arteriovenous malformation (AVM), and one (0.9%) renal stent placement.
Of these 108 cases, the Angio-Seals were used in 42 cases (the Angio-Seal group), and the Closer S was used in 66 cases (the Closer S group). There was no difference between the groups according the vascular closure devices in terms of the demographic findings, the risk factors and the coagulation profiles (Table 2). Immediate hemostasis was achieved in 95.2% of the Angio-Seal group and in 89.4% of the Closer S group. Total success was achieved in 92.9% of the Angio-Seal group and in 84.8% of the Closer S group. Early complications occurred in four patients. These included two episodes of active rebleeding that required manual compression for at least 10 minutes (2 in the Closer S group), and two episodes of minor oozing that required prolonged strict bed rest for at least an additional two hours (1 in the Angio-Seal group and 1 in the Closer S group). There was no late complication. In the drug group, the successful hemostasis rate was higher for the collagen plug devices than for the suture-mediated devices; however, the difference was not statistically significant (Table 3). Forty-one patients (38%) in the drug group received systemic anticoagulation therapy after the procedures.
All of the cases that exhibited early minor complications were the heparin-only cases. The mean dose of heparin that was administered in these four cases was 3,750±500 units, while the mean heparin dose in all the heparin-only cases was 4,238±1,287.6 units. The 6-F arterial sheath was used for three of these cases, and the 8 F was used for one of the four cases in which complications occurred. Out of the total 108 cases, the 5-F arterial sheath was used in three cases (2.8%), the 6-F was used in 87 cases (80.6%), the 7 F was used in three cases (2.8%), and the 8 F was used in 15 cases (13.9%). Among the four patients with minor complications, only one patient received systemic anticoagulation after the procedure. We determined that there was no association between the complications and the heparin dose, the history of multiple punctures, the coagulation profile and the systemic coagulation that was used after the procedures in the drug group (Table 4).
Both the no-drug and drug groups showed no significant differences in the demographic findings and the risk factors. However, for the drug group, the coagulation profile was better than that for the no-drug group (p < 0.01) (Table 5).
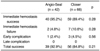
Immediate hemostasis was achieved in 92.9% of the no-drug group and in 91.7% of the drug group. Total success was achieved in 90.8% of the no-drug group and in 88.0% of the drug group. There was no statistically significant differences between these two groups (p = 0.34). Although the early complication rate tended to be higher in the cases that were administered with drugs, this difference was not statistically significant (p = 0.10) (Table 6).
The management of femoral artery access sites following percutaneous intervention continues to be an important aspect of diagnostic and therapeutic interventions. Inducing adequate hemostasis must be done safely, and this has traditionally been performed by manual compression or by using clamp-like compression devices (15-17).
Multiple variables have been associated with the incidence of access-site complications in the patients who have undergone percutaneous vascular interventions; these variables include advanced age, diabetes, hypertension and cigarette smoking (18). In addition, many studies have suggested that concomitant anticoagulation or antiplatelet therapy tends to increase the risks of complication in the vascular access sites when only manual compression is used (3, 19-23).
A few studies have recently suggested that vascular closure devices are both safe and effective for the management of arterial access sites in the patients who receive anticoagulant or antiplatelet therapy, as compared with manual compression (14, 20). Applegate et al. (24) have reported a major vascular complication rate of 0.9% for the combined closure-device group and 1.3% for the manual-pressure group in the patients who received glycoprotein IIb/IIIa inhibitor therapy. Duffin et al. (25) have suggested that routine abciximab use by itself was not associated with vascular complications. Sesana et al. (26) have recently reported a vascular complication rate of 2.5% for the patients treated with the Angio-Seal device, and a rate of 3.4% for the patients who were treated with the Prostar device; these rates closely approximated the rates that we have reported here. These investigators also concluded that the use of the glycoprotein IIb/IIIa antagonist, abciximab, was not associated with an increased risk of vascular complications. The principal finding of our study is that femoral arterial closures can be performed both safely and effectively with the currently available vascular closure devices in the patients who received heparin, thrombolytics or abciximab during their procedures.
The reported success rates for the collagen plug and suture-mediated closure devices have ranged from 90% to 100% (8, 27). In our baseline study, immediate hemostasis was achieved in 95.2% of the 961 cases using the Angio-Seal, and in 89.5% of the 715 cases using the Closer S (p < 0.001); total success was achieved in 92.6% of the cases using the Angio-Seal, and in 87.8% of the cases using the Closer S (p < 0.01). Thus, the use of the Angio-Seal appeared to be slightly more effective than that of the Closer S, with regard to inducing hemostasis at the femoral access site.
The immediate hemostasis rates were 92.9% (1,456/1,568) in the no-drug group and 91.7% (99/108) in the drug group. The total success rates were 90.8% (1,423/1,568) in the no-drug group and 88.0% (95/108) in the drug group. However, no statistically significant differences were determined to exist between these two groups according to the medications they received. The early complication rates tended to be higher when the patients had received the medications, but this difference was also not significant statistically. The higher dose of the medications, a history of multiple punctures, the coagulation function and further systemic anticoagulation after the procedures appeared to have no effect on the complication rates, based on our experiences.
There are two limitations of our study. First, our data showed a statistically significant difference in the coagulation profile between the no-drug group and the drug group. Among the no-drug group (1,568 cases), 808 (51.5%) cases with liver cirrhosis had undergone transarterial chemoembolization for the treatment of hepatic tumors, and these patients had impaired hepatic function. Moreover, stricter indications for the procedures were applied to the drug group. These factors may have contributed to these unexpected results. In our supplementary study on the patients with early or late complications, 33.3% (22/33) of the no-drug group and none of the patients of the drug group showed an abnormal platelet count (p < 0.30). Further, all the patients with early or late complications showed a normal prothrombin time before the procedures. So, we considered that our results were not influenced by these differences. Second, our study is limited by the relatively small size of the drug group; heparin, glycoprotein IIb/IIIa inhibitor or thrombolytics was administered to only 6.4% of the total 1,656 cases. For example, we described that the immediate hemostasis and total success rates were not statistically different between the two closure device groups in the drug group. Indeed, the small sample size for the drug group contributed to these varying results, as compared to the baseline outcomes. A larger comparative study that includes more patients treated with drugs during the procedures is needed.
In conclusion, the use of anticoagulants, antiplatelet agents or thrombolytics during the procedures does not significantly affect the immediate/total success and the complication rates, and these findings are regardless of the types of vascular closure devices that are used. Therefore, we can conclude that arterial closure of femoral access sites using vascular closure devices constitutes a safe and effective therapeutic treatment, even for the patients who receive anticoagulant, abciximab, or thrombolytics during the procedures.
Figures and Tables
Table 2
Comparison of the Demographic Findings, Risk Factors, and Coagulation Profiles in the Drug Group
References
1. Kahn ZM, Kumar M, Hollander G, Frankel R. Safety and efficacy of the Perclose suture-mediated closure device after diagnostic and interventional catheterizations in a large consecutive population. Catheter Cardiovasc Interv. 2002. 55:8–13.
2. Kent KC, McArdle CR, Kennedy B, Baim DS, Anninos E, Skillman JJ. A prospective study of the clinical outcome of femoral pseudoaneurysms and arteriovenous fistulas induced by arterial puncture. J Vasc Surg. 1993. 17:125–131. discussion 131-123.
3. Kresowik TF, Khoury MD, Miller BV, Winniford MD, Shamma AR, Sharp WJ, et al. A prospective study of the incidence and natural history of femoral vascular complications after percutaneous transluminal coronary angioplasty. J Vasc Surg. 1991. 13:328–333. discussion 333-325.
4. Rickli H, Unterweger M, Sutsch G, Brunner-La Rocca HP, Sagmeister M, Ammann P, et al. Comparison of costs and safety of a suture-mediated closure device with conventional manual compression after coronary artery interventions. Catheter Cardiovasc Interv. 2002. 57:297–302.
5. Katzenschlager R, Ugurluoglu A, Ahmadi A, Hulsmann M, Koppensteiner R, Larch E, et al. Incidence of pseudoaneurysm after diagnostic and therapeutic angiography. Radiology. 1995. 195:463–466.
6. Gerckens U, Cattelaens N, Lampe EG, Grube E. Management of arterial puncture site after catheterization procedures: evaluating a suture-mediated closure device. Am J Cardiol. 1999. 83:1658–1663.
7. Gerckens U, Cattelaens N, Muller R, Lampe EG, Grube E. Percutaneous suture of femoral artery access sites after diagnostic heart catheterization and or coronary intervention. Safety and effectiveness of a new arterial suture technic. Herz. 1998. 23:27–34.
8. Baim DS, Knopf WD, Hinohara T, Schwarten DE, Schatz RA, Pinkerton CA, et al. Suture-mediated closure of the femoral access site after cardiac catheterization: results of the suture to ambulate aNd discharge (STAND I and STAND II) trials. Am J Cardiol. 2000. 85:864–869.
9. Wetter DR, Rickli H, von Smekal A, Amann FW. Early sheath removal after coronary artery interventions with use of a suture-mediated closure device: clinical outcome and results of Doppler US evaluation. J Vasc Interv Radiol. 2000. 11:1033–1037.
10. Koreny M, Riedmuller E, Nikfardjam M, Siostrzonek P, Mullner M. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA. 2004. 291:350–357.
11. Michalis LK, Rees MR, Patsouras D, Katsouras CS, Goudevenos J, Pappas S, et al. A prospective randomized trial comparing the safety and efficacy of three commercially available closure devices (Angio-Seal, Vasoseal and Duett). Cardiovasc Intervent Radiol. 2002. 25:423–429.
12. O'Sullivan GJ, Buckenham TM, Belli AM. The use of the angio-seal haemostatic puncture closure device in high risk patients. Clin Radiol. 1999. 54:51–55.
13. Grollman JH Jr. Percutaneous arterial access closure: now do we have the be all and end all? not yet! Catheter Cardiovasc Interv. 2000. 49:148–149.
14. Assali AR, Sdringola S, Moustapha A, Ghani M, Salloum J, Schroth G, et al. Outcome of access site in patients treated with platelet glycoprotein IIb/IIIa inhibitors in the era of closure devices. Catheter Cardiovasc Interv. 2003. 58:1–5.
15. Simon A, Bumgarner B, Clark K, Israel S, Bogart MA. Manual versus mechanical compression for femoral artery hemostasis after cardiac catheterization. Am J Crit Care. 1998. 7:308–313.
16. Bogart MA. Time to hemostasis: a comparison of manual versus mechanical compression of the femoral artery. Am J Crit Care. 1995. 4:149–156.
17. Colapinto RF, Harty PW. Femoral artery compression device for outpatient angiography. Radiology. 1988. 166:890–891.
18. Heintzen MP, Strauer BE. Peripheral arterial complications after heart catheterization. Herz. 1998. 23:4–20.
19. Pache J, Kastrati A, Mehilli J, Gawaz M, Neumann FJ, Seyfarth M, et al. Clopidogrel therapy in patients undergoing coronary stenting: value of a high-loading-dose regimen. Catheter Cardiovasc Interv. 2002. 55:436–441.
20. Resnic FS, Blake GJ, Ohno-Machado L, Selwyn AP, Popma JJ, Rogers C. Vascular closure devices and the risk of vascular complications after percutaneous coronary intervention in patients receiving glycoprotein IIb-IIIa inhibitors. Am J Cardiol. 2001. 88:493–496.
21. Popma JJ, Satler LF, Pichard AD, Kent KM, Campbell A, Chuang YC, et al. Vascular complications after balloon and new device angioplasty. Circulation. 1993. 88:1569–1578.
22. Omoigui NA, Califf RM, Pieper K, Keeler G, O'Hanesian MA, Berdan LG, et al. Peripheral vascular complications in the Coronary Angioplasty Versus Excisional Atherectomy Trial (CAVEAT-I). J Am Coll Cardiol. 1995. 26:922–930.
23. Lumsden AB, Miller JM, Kosinski AS, Allen RC, Dodson TF, Salam AA, et al. A prospective evaluation of surgically treated groin complications following percutaneous cardiac procedures. Am Surg. 1994. 60:132–137.
24. Applegate RJ, Grabarczyk MA, Little WC, Craven T, Walkup M, Kahl FR, et al. Vascular closure devices in patients treated with anticoagulation and IIb/IIIa receptor inhibitors during percutaneous revascularization. J Am Coll Cardiol. 2002. 40:78–83.
25. Duffin DC, Muhlestein JB, Allisson SB, Horne BD, Fowles RE, Sorensen SG, et al. Femoral arterial puncture management after percutaneous coronary procedures: a comparison of clinical outcomes and patient satisfaction between manual compression and two different vascular closure devices. J Invasive Cardiol. 2001. 13:354–362.
26. Sesana M, Vaghetti M, Albiero R, Corvaja N, Martini G, Sivieri G, et al. Effectiveness and complications of vascular access closure devices after interventional procedures. J Invasive Cardiol. 2000. 12:395–399.
27. Carey D, Martin JR, Moore CA, Valentine MC, Nygaard TW. Complications of femoral artery closure devices. Catheter Cardiovasc Interv. 2001. 52:3–7. discussion 8.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download