Abstract
We present here a case in which functional MR imaging (fMRI) was done for a patient who developed retrograde psychogenic amnesia for a four year period of her life history after a severe stressful event. We performed the fMRI study for a face recognition task using stimulation with three kinds of face photographs: recognizable familiar faces, unrecognizable friends' faces due to the psychogenic amnesia, and unfamiliar control faces. Different activation patterns between the recognizable faces and unrecognizable faces were found in the limbic area, and especially in the amygdala and hippocampus.
Psychogenic amnesia is a dissociative disorder characterized by a sudden memory loss that concerns important personal information and there is usually selective retrograde amnesia too, but this condition is not due to an organic mental disorder. The amnesia may follow severe psychological stress or it may be an unconscious response to an internal conflict or to an intolerable life situation (1). The patient with psychogenic amnesia usually has difficulties for face recognition and the affective response to faces in relation to the faces' familiarity during the selective amnesic period (2).
The human memory system is a complex series of functional neuroanatomical events (3). Some experimental data have been presented to elucidate the brain changes associated with psychogenic amnesia (4-6). However, to the best of our knowledge, there has been no report of performing functional MR imaging (fMRI) during psychogenic amnesic states. We report here on a case of a patient's functional MR imaging (fMRI), and the patient had selective amnesia after a severe psychological stress and further, this patient had no evidence of an organic disease background.
A 22-year-old, right-handed, unmarried woman was admitted to the psychiatric unit due to her selective retrograde amnesia that occurred after a severe stressful event. She could remember nothing of the autobiographical events for the preceding four years, that is, her university life period. She was aware only of a hallucinatory vision of a parking lot in which she was kidnapped and violated by a masked robber. However, she could recount in detail the events of her high school life and also the events of her life before that. There were no neurological signs, no suspicion of head trauma and no epileptic discharge on the electroencephalography. Her brain MRI was normal.
To examine the underlying brain functional disturbances associated with psychogenic amnesia, we performed a fMRI study for a face recognition task. The patient viewed three kinds of face photographs: recognizable faces of familiar high school friends, unrecognizable faces of familiar university friends, and unfamiliar control faces. The patient was instructed to try to recognize the faces that were shown to her. All faces were presented to the patient through a mirror located at the top of the head coil that received the face photographs from outside of the magnetic room during the fMRI procedure.
The T1-weighted images (time to repeat/echo time/flip angle = 500 ms/50 ms/90°) were obtained for anatomical localization by using a 1.5 T MRI scanner (GE Medical System, Horizon Milwaukee, WI, USA). The BOLD-contrast fMRI images were acquired from 11 slices that covered the limbic areas with using gradient-echo EPI (time to repeat/echo time/flip angle = 3000 ms/50 ms/90°). Post-processing of images was performed with MATLAB (MathWorks, Natick, MA, USA) and SPM99 (Statistical Paramatric Mapping software; Wellcom Department of Cognitive Neurology, London, UK). The SPM99 software was used for image realignment, normalization, smoothing and to create the statistical maps of the significant relative changes in the regional blood oxygenation level-dependent (BOLD) response.
The blocked design fMRI paradigm, which was preceded by three dummy scans to allow the magnetic resonance signal to reach a steady state, was comprised of seven repetitions with nine seconds of rest (fixation cross), and six repetitions of a nine seconds activation state (three kinds of photograph that were randomly comprised of three seconds of recognizable faces of high school friends, three seconds of unrecognizable faces of university friends, and three seconds of unfamiliar control faces). A correlation coefficient algorithm was used to correlate the time course data sets with the periodic boxcar function. Three different subsets of the activation periods were independently compared with those of the rest periods. The BOLD signals were estimated for the comparison of whole brain signals across all the experimental conditions. Specific effects were tested for by applying appropriate linear contrasts to the parameter estimates for each condition, and this resulted in a Z-score for each voxel. The voxels were identified as being significant only if they passed a height threshold of Z = 3.5 and they belonged to a cluster of at least 30 activated voxels. The data were analyzed for the main effects of each stimulation: the recognizable faces of high school friends, the unrecognizable faces of university friends and the unfamiliar control faces.
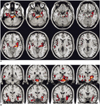
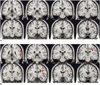
For the recognizable condition, different patterns of brain activation were found as compared with the unrecognizable condition. The functional maps of the representative brain slices demonstrated differences for activation in the limbic areas: the amygdala, hippocampus, parahippocampal gyrus and insula. Especially, the activation of the amygdala and hippocampus was significantly greater during the stimulation with recognizable faces of high school friends (Fig. 1). However, the activation patterns stimulated with the unrecognizable faces of university friends and the unfamiliar control faces were similar to each other. They showed significantly less activation in the limbic area than the recognizable faces (Fig. 2).
In our case, we found significantly different patterns of activation in the limbic system, especially in the amygdala and hippocampus areas, during the stimulation with recognizable faces of high school friends and the unrecognizable faces of university friends. Well-known faces have the potential to elicit retrieval of affective responses and personal episodic incidents, and they may evoke activation of the limbic system, especially the amygdala (7). The amygdala is located in the anterior medial portion of the temporal lobe, and it is connected with the hippocampal formation and with further cortical and subcortical structures. The amygdala is primarily involved in the acquisition and expression of emotional memories (8). Presumably, this patient has an abnormality for the expression of emotional memories during the amnesic period.
Emotional overflow could have resulted in the memory impairments. For instance, traumatic stress may lead to remarkable encoding and retrieval disturbance, and this is probably due to the increased release of stress-related hormones such as glucocorticoids. The intense release of glucocorticoids may result in morphologic and functional alterations of the limbic structures, especially in mediotemporal regions. These can cause broad changes in the neurotransmitter systems of the brain. In a recent study, the relation between the traumatic stress and functional brain alterations was described in terms of "psychogenic amnesia" (9).
The hippocampal region is concerned with explicit judgments that are related to the identification of faces. The lack of activation in the hippocampal region might be related to an abnormality in the explicit components of the face judgment (10). This hippocampal abnormality may be related to the symptoms of psychogenic amnesia.
In conclusion, this fMRI study could give objective evidence and illustrate that a psychogenic amnesic patient has an abnormality in the retrieval of emotional memories during the amnesic period. These findings suggest that changes in the limbic functions are related to the symptoms of psychogenic amnesia.
Figures and Tables
References
1. Kaplan HI, Sadock BJ. Comprehensive textbook of psychiatry. 1995. 6th ed. New York: William & Wilkins;650–653.
2. Pujol M, Kopelman MD. Psychogenic amnesia. Practical Neurology. 2003. 3:292–299.
3. Lee SH. Brain mechanism of memory and psychiatry. J Korean Neuropsychiatr Assoc. 1998. 37:14–37.
4. Markowitsch HJ. Psychogenic amnesia. Neuroimage. 2003. 20:132–138.
5. Yasuno F, Nishigawa T, Nakagawa Y, Ikejiri Y, Tokunaga H, Mizuta I, et al. Functional anatomical study of psychogenic amnesia. Psychiatry Res. 2000. 99:43–57.
6. Markowitsch HJ, Calabrese P, Fink GR, Durwen HF, Kessler J, Harting C, et al. Impaired episodic memory retrieval in a case of probable psychogenic amnesia. Psychiatry Res. 1997. 74:119–126.
7. Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception: the neural basis of normal emotion perception. Biol Psychiatry. 2003. 54:504–514.
8. Ledoux JE, Muller J. Emotional memory and psychopathology. Philos Trans R Soc Lond B Biol Sci. 1997. 352:1719–1726.
9. Calabrese P, Markowitsch HJ. Memory and brain: neurobiological correlates of memory disturbances. Fortschr Neurol Psychiatr. 2003. 71:211–219.
10. Kapur N, Friston KJ, Young A, Frith CD, Frackowiak RS. Activation of human hippocampal formation during memory for faces: a PET study. Cortex. 1995. 31:99–108.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download