Abstract
Objective
To characterize the imaging features on gray-scale and contrast-enhanced color Doppler US images which differentiate renal ischemia from renal infarction.
Materials and Methods
The segmental renal arteries of eight healthy rabbits were surgically ligated. In four of these rabbits, the ligated renal artery was released 60 minutes after arterial occlusion to cause transient ischemia. In the remaining four rabbits, the arterial ligation was retained to cause a permanent infarction. The gray-scale and contrast-enhanced color Doppler US imaging features of the involved renal parenchyma of both ischemia and infarction groups were compared with respect to the presence or absence of parenchymal swelling, echogenicity changes, tissue loss and perfusion defects.
Results
Parenchyma swelling, echogenic changes, tissue loss and perfusion defects were found to be more extensive in the infarction than the ischemia group. The hyperechoic areas reperfused with blood flow recovered normal echogenicity and perfusion, whereas the hyperechoic areas without reperfusion became renal infarcts.
Many studies, using various imaging modalities, including US, CT and MRI, have described the imaging features of renal infarction (1-10). However, there have been no reports concerning the imaging features of transient renal ischemia, which differs pathologically from those of renal infarction. Moreover, although these two lesions appear clinically alike, renal ischemia may be a reversible change. Therefore, it is of considerable importance that the differential imaging features of renal ischemia and renal infarction are documented. The purpose of this study was to identify those differential imaging features capable of differentiating renal ischemia from renal infarction on gray-scale and color Doppler US images using an experimental model.
Eight male New Zealand White rabbits, weighing approximately 3 kilograms each, were included in this study. These rabbits were anesthetized with an intravenous injection of ketamin (40 mg/kg) and xylazine (5 mg/kg), and placed in the right decubitus position; thus, allowing a retroperitoneal approach to the left renal hilum. The left flank was incised, the retroperitoneal space dissected, and the left renal hilum exposed through the posteromedial aspect of the kidney. The segmental left renal artery supplying the lower pole was isolated and ligated with surgical silk. The incised flank was immediately closed in four rabbits following the surgical procedure in order to cause a permanent segmental infarction. In the remaining four rabbits, the segmental arterial obstruction was released 60 minutes later after ligation, to cause transient renal ischemia and reperfusion, and then the incised flank closed. A total of 100 mg of ampicillin sodium was intravenously injected to prevent infection.
Gray-scale and contrast-enhanced color Doppler US imaging were performed using a Sequoia 512 system (Siemens Medical Solution, Mountain View, CA, USA), equipped with a linear array transducer of 8-15 MHz. The Doppler US gain was gradually increased until background noise was observed, which was then reduced just enough to remove the noise from the appearance of the kidney. Intravenous anesthesia was performed with ketamin (40 mg/kg) and xylazine (5 mg/kg) prior to the US scan.
SH U 508A (Levovist; Schering AG, Berlin, Germany) was used as the US contrast agent, which consisted of galactose microparticles (99.9%) and palmitic acid (0.1%). A total of 300 mg of the US contrast agent (100 mg per kilogram) was intravenously administered as a manual bolus injection.
US images were obtained on preoperative day one, immediate after wound repair, and when scheduled on postoperative days (POD) one, three, seven, 14 and 28. Immediately after the final US imaging on POD 28, all rabbits were sacrificed with an overdose of intravenous thiopental sodium, and the left kidneys harvested for histological examination, which were fixed in formalin and stained with hematoxylin and eosin.
The imaging features of the involved renal parenchyma were compared between the renal ischemia and infarction groups, on gray-scale US images, for the presence or absence of parenchymal swelling, echogenicity changes and tissue loss. Parenchymal swelling was graded as follows: mild (mildly swollen with no obliteration of renal sinus), moderate (moderately swollen enough to compress the renal sinus by less than half of the normal sinus thickness) and severe (severely swollen enough to compress the renal sinus by more than half of normal sinus thickness). Echogenicity changes or tissue loss were graded as follows: mild (echogenicity change or tissue loss of less than one third of the lower polar parenchyma), moderate (echogenicity change or tissue loss between one third and two thirds of the lower polar parenchyma) and severe (echogenicity change or tissue loss of more than two thirds of the lower polar parenchyma).
On contrast-enhanced color Doppler US images, the perfusion defects of the involved renal parenchyma were compared between the ischemia and infarction groups, and graded as follows: mild (perfusion defect of less than one third of the lower polar parenchyma), moderate (perfusion defect between one third and two thirds of the lower polar parenchyma) and severe (perfusion defect of more than two thirds of the lower polar parenchyma).
Surgical ligations of the segmental renal arteries were all successfully performed. All rabbits, with the exception of one, survived until POD 28, the last day of US imaging. Various abscesses occurred in the perirenal area or in the left flank wall, but these responded to antibiotics.
Table 1 summarizes the imaging features of the gray-scale US in the ischemic and infarction groups. Parenchymal swellings in the ischemic and infarction groups peaked on POD three and seven, respectively, by normalized by POD 14. The involved parenchyma was more swollen in the infarction group (Fig. 1).
Renal ischemic and infarction areas showed hypoechoic parenchyma before POD seven, which were then replaced with a hyperechoic area. The hyperechoic area in the renal ischemic group was greatest at POD seven, and then gradually reduced until POD 28 (Fig. 2). A hyperechoic area in the infarction group was observed at POD seven, which did not alter until POD 28 (Fig. 3). Echogenicity changes of the involved parenchyma were more extensive in the infarction group.
Parenchymal tissue loss was observed in both groups at POD 14, with the infarction group showing more tissue loss than the ischemia group.
Table 2 summarizes the perfusion defect changes in the two groups according to the postoperative day. The ischemic group showed the most extensive perfusion defect area on POD one. This imaging feature improved from POD three, and appeared as a mild perfusion defect on POD 28 (Fig. 2). Normal renal perfusion of the lower polar parenchyma did not fully recover in the ischemic group. However, the hyperechoic areas that showed blood flow recovery after reperfusion regained normal echogenicity and perfusion, but the hyperechoic areas that were not reperfused became infarcted. The rabbits that underwent renal infarction showed complete perfusion defect of the lower polar parenchyma and no recovery of renal perfusion at POD 28 (Fig. 3).
The pathological specimens obtained from the ischemic group showed minimal tissue loss of the lower polar parenchyma, as a result of coagulation necrosis of the renal tubules and glomeruli. However, those obtained from the infarction group showed severe tissue loss and hemorrhage, as a result of extensive necrosis of the lower polar parenchyma.
Many clinical and experimental studies have described the findings of renal infarction with the use of US, CT or MRI (1-10). Renal infarction is usually diagnosed based on the CT findings, including a sharply demarcated area of poor contrast enhancement, a hyperdense cortical rim and subcapsular fluid collection (3, 4, 10). MRI can also be useful for the detection of renal infarction, and in this case usually appears as areas of low signal intensity on both T1- and T2-weighted MR images, and as a region of poor contrast enhancement on contrast-enhanced MR images (1, 2, 6, 7). Doppler US has the ability to depict focal perfusion defects by clearly visualizing fine small vessels in the renal parenchyma (5, 9). Moreover, gray-scale US can show echogenicity changes in the infarction areas, including hypoechoic and hyperechoic involved parenchyma in the early and late stages of the infarction, respectively (8). However, previous studies have primarily focused on the renal infarction, so have not addressed the transient renal ischemic changes. Moreover, these must be differentiated from renal infarction, as renal ischemia is theoretically reversible; whereas, renal infarction is not. Therefore, it is of some great importance to obtain imaging features that are able to differentiate renal ischemia from renal infarction during evaluation of the management and prognosis of patients with a renal vascular impairment.
In the present study, gray-scale US and contrast-enhanced color Doppler US were able to depict potentially differentiating features of renal ischemia and renal infarction. During the early POD, parenchymal swelling, hypoechogenicity and reduced renal perfusion were common US imaging features in both the ischemic and renal infarction groups. The renal parenchyma of both groups showed postoperative echogenicity changes from hypoechoigenicity to hyperechogenicity. However, the reperfused hyperechoic areas, unlike the infarct areas, returned to being normal parenchyma. This imaging feature should be considered a differential US imaging feature of renal ischemia and renal infarction. On the contrary, hyperechoic areas without reperfusion proved irreversible, and became infarcted. Spies et al. reported that renal parenchymal hyperechogenicity followed hypoechogenicity after arterial occlusion, and these regions corresponded pathologically to infarction (8). However, our study demonstrated that reperfused hyperechoic renal parenchyma was able to recover normal echogenicity and perfusion. The hyperechoic area corresponded pathologically to an ischemic penumbra area, which does not determined whether this lesion returned to normal or became infarcted. This should depend on the presence or absence of arterial reperfusion to the renal ischemic area.
We determined the optimal ischemic duration based on the study of Regan et al. (11), who reported that renal infarction occurs when renal artery was occluded for more than 90 minutes. In our pilot study, 60 minutes was found to be the optimal ischemic duration for depiction of renal ischemia and infarction on US. 30 minute duration failed to visualize the ischemic or infarction area on US because the involved area was too small; whereas, 90 minute duration made it impossible to differentiate renal ischemia from infarction as all involved area had become totally infarcted.
Our study had several limitations; first, a relatively small number of cases were included. Thus, further investigation will be necessary on a larger study population. Second, US imaging features at the early stage of renal ischemia and infarction did not correlate with those of the pathological examination. In fact, pathological correlations were only available from the last US image. Third, subcapsular rim enhancement was not observed on the contrast-enhanced color Doppler US. This was why the subcapsular arteries might have been injured when the left kidneys were isolated from the perirenal space.
In conclusion, gray-scale and contrast-enhanced color Doppler US were found to have the potential to depict various imaging features as a result of renal ischemia and renal infarction. A hyperechoic region, with normal blood flow, may be a reversible US feature to provide a clue to the differentiation of renal ischemia from renal infarction.
Figures and Tables
Fig. 1
Comparison of the parenchyma swelling between renal ischemia and infarction on postoperative day one.
A. Longitudinal image of gray-scale US shows no or only mild parenchymal swelling (arrows), without echogenicity change, as a result of transient renal ischemia.
B. Longitudinal image of contrast-enhanced color Doppler US shows moderate or severe swelling (arrows) of the hypoechoic parenchyma as a result of renal infarction. (Figure B has been permitted by the Journal of Korean Society of Ultrasound in Medicine 2004;23:137-145)
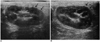
Fig. 2
Gray-scale and contrast-enhanced color Doppler US images of renal ischemia on postoperative day 28.
A. Transverse image of gray-scale US shows a wedge-shaped hyperechoic area (arrows) in the lower pole of the left kidney.
B. Transverse image of contrast-enhanced color Doppler US shows reperfusion (arrows) of blood flow in the hyperechoic area.
C. Transverse section of a pathologic specimen (×1, haematoxylin and eosin staining) shows a normal parenchyma (single arrow), with reperfusion between the infarction areas (double arrows) without reperfusion.
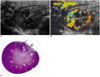
Fig. 3
Contrast-enhanced color Doppler US images of renal infarction on postoperative day 28.
A. Longitudinal image of contrast-enhanced color Doppler US shows a complete perfusion defect (arrows) in the hyperechoic lower polar parenchyma of the left kidney, which is severely atrophic.
B. Longitudinal section of a pathologic specimen shows atrophic parenchyma (arrows), with infarction, which correlated well with the US findings. (Figure B has been permitted by the Journal of Korean Society of Ultrasound in Medicine 2004;23:137-145)

References
1. Choo SW, Kim SH, Jeong YG, Shin YM, Kim JS, Han MC. MR imaging of segmental renal infarction: an experimental study. Clin Radiol. 1997. 52:65–68.
2. Choyke PL, Pollack HM. The role of MRI in diseases of the kidney. Radiol Clin North Am. 1988. 26:617–631.
3. Glazer GM, Francis IR, Brady TM, Teng SS. Computed tomography of renal infarction: clinical and experimental observations. AJR Am J Roentgenol. 1983. 140:721–727.
4. Hilton S, Bosniak MA, Raghavendra BN, Subramanyam BR, Rothberg M, Megibow AJ. CT findings in acute renal infarction. Urol Radiol. 1984. 6:158–163.
5. Huber S, Steinbach R, Sommer O, Zuna I, Czembirek H, Delorme S. Contrast-enhanced power Doppler harmonic imaging-influence on visualization of renal vasculature. Ultrasound Med Biol. 2000. 26:1109–1115.
6. Ishikawa I, Masuzaki S, Saito T, Yuri T, Shinoda A, Tsujigiwa M. Magnetic resonance imaging in renal infarction and ischemia. Nephron. 1989. 51:99–102.
7. Kim SH, Park JH, Han JK, Han MC, Kim S, Lee JS. Infarction of the kidney: role of contrast enhanced MRI. J Comput Assist Tomogr. 1992. 16:924–928.
8. Spies JB, Hricak H, Slemmer TM, Zeineh S, Alpers CE, Zayat P, et al. Sonographic evaluation of experimental acute renal arterial occlusion in dogs. AJR Am J Roentgenol. 1984. 142:341–346.
9. Taylor GA, Ecklund K, Dunning PS. Renal cortical perfusion in rabbits: visualization with color amplitude imaging and an experimental microbubble-based US contrast agent. Radiology. 1996. 201:125–129.
10. Wong WS, Moss AA, Federle MP, Cochran ST, London SS. Renal infarction: CT diagnosis and correlation between CT findings and etiologies. Radiology. 1984. 150:201–205.
11. Regan FC, Crabtree EG. Renal infarction: a clinical and possible surgical entity. J Urol. 1948. 59:981–1018.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download