INTRODUCTION
Ballism is a repetitive, constantly varying, large-amplitude involuntary movement of the proximal portion of the extremities. Chorea is a similar disorder, but the movements are more continuous, random, and jerky, and they affect the distal portion of the extremities (1). Hemichorea-hemiballism (HCHB) is a hyperkinetic disorder characterized by unilateral, involuntary movements, and it is usually caused by lesions in the contralateral corpus striatum (1). HCHB actually describes a clinical spectrum, in which hemiballism often evolves into hemichorea (2). It can result from various conditions, including vascular insufficiency, infection, drugs, metabolic disorders, neurodegenerative disorders, and brain tumors. HCHB may also represent the first clinical feature of hyperglycemia. Recognition of hyperglycemia-induced HCHB is important because it is treatable, and when treated, it usually has a good prognosis (3). Several previous studies on patients with hyperglycemia-associated HCHB identified a characteristic high signal intensity in the contralateral corpus striatum using T1-weighted MRI (1). However, to the best of our knowledge, temporal lobe and midbrain involvement in HCHB associated with hyperglycemia has been very rarely described. Here, we describe a case of HCHB associated with nonketotic hyperglycemia, with involvement of the temporal lobe and midbrain, which was diagnosed by MRI.
CASE REPORT
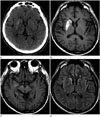
A 51-year-old man was admitted with a history of involuntary movements of the left leg for 1 month, which had subsequently progressed to the left arm. Movements resolved during sleep, but they were almost continuous during wakefulness. He did not demonstrate any other neurologic deficit, such as hemiparesis or sensory change. He had a history of diabetes mellitus, and he reported recent poor oral intake and weight loss. He also presented with poorly controlled hyperglycemia. He did not take any medication that could induce chorea or ballismus, nor did he have a family history of any kind of neurologic disorders, including movement disorders. On admission, he presented with a blood glucose level of 365 mg/dL and glycosylated hemoglobin A1c of 13.9%. Although urine testing for ketone bodies was not performed, no ketones were found in his serum and his serum pH was normal. His corrected sodium concentration was 140.7 mmoL/L, and estimated serum osmolality was 303 mOsm/kg. Other routine blood tests yielded normal results. Neurologic examination also produced normal results, except for unilateral involuntary movements of the left extremities. Non-contrast CT showed a hyperdense area in the right corpus striatum and medial temporal lobe. T1-weighted brain MRI showed increased signal intensity in the right corpus striatum, medial temporal lobe, and cerebral peduncle in the midbrain. Fluid-attenuated inversion recovery MR images demonstrated increased signal intensity in the right corpus striatum and temporal lobe, but sparing of the anterior limb of the right internal capsule (Fig. 1). Although HCHB-related movements decreased in terms of severity after haloperidol administration (12 mg/day), and hyperglycemia was controlled with insulin and metformin, these conditions persisted at discharge.
DISCUSSION
Nonketotic hyperglycemia can cause various movement disorders (4). Most of the cases of HCHB-associated hyperglycemia have been reported in older patients with type 2 diabetes mellitus and nonketotic hyperglycemia. In addition, most of the affected patients are female and East Asians, leading to a hypothesis of dopamine hypersensitivity or a possible genetic predisposition (14). In contrast to the other reported cases, our case was of a middle-aged Korean man with diabetes mellitus.
Diagnosis of HCHB associated with hyperglycemia is based on chorea, nonketotic hyperglycemia, and striatal high signal intensity on T1-weighted MRI. Our case presented with high signal intensity in the right corpus striatum. Basal ganglia abnormalities represented as high signal intensity on T1-weighted images were frequently observed in previous reports, particularly in the putamen and caudate, and sometimes bilaterally (2345). However, the anterior limb of the internal capsule was characteristically spared, as noted in our study. Notably, HCHB asso-ciated with hyperglycemia is usually localized to the putamen and/or caudate nucleus, but our patient also presented with involvement of the medial temporal lobe and cerebral peduncle in the midbrain. Only a few prior cases have reported an abnormal signal intensity that extended to the globus pallidus and up to the medial part of the cerebral peduncle in the midbrain, following the striatonigral pathway (2).
The underlying pathophysiology is not fully understood, but a range of theories have been suggested, including cerebral vascular insufficiency, neuronal dysfunction secondary to hyperosmolar or hyperglycemic insult, abundant gemistocytes, petechial hemorrhage, hyperviscosity, and decreased synthesis of γ-amin-obutyric acid (GABA) and acetylcholine secondary to metabolic changes (16). A previous autopsy study (2) discerned that a hyperintense lesion in the putamen was due to abundant gemistocytes located along the axons that had persisted for several years. Other reports suggested that decreased blood flow caused impairment of GABAergic neurons in the putamen, allowing hyperactivation of dopaminergic neurons (3678). However, the most recently accepted mechanism is petechial hemorrhage, which is induced when hyperglycemia disrupts the blood brain barrier and causes transient ischemia in vulnerable striatal neurons (69).
In these cases, HCHB can be rapidly resolved by glycemic control and the use of neuroleptics. Despite the control of hyperglycemia, involuntary movements can persist in some cases. These patients usually show a good response to conventional neuroleptics, such as haloperidol, perphenazine, and chlorpromazine. The signal change in the corpus striatum may decrease, or it may persist for months or years after symptom improvement (10). We observed symptomatic relief after glycemic control and the use of haloperidol in our patient. The prognosis of HCHB associated with nonketotic hyperglycemia is generally good, although it worsens if treatment is delayed.
In conclusion, hyperintensity in the putamen and/or the caudate is seen in nonketotic hyperglycemic HCHB patients, and it may extend to the medial temporal lobe and the medial part of the cerebral peduncle. These findings on MRI may be helpful for early diagnosis and treatment of HCHB associated with nonketotic hyperglycemia.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download