Abstract
Purpose
To compare the mid-term follow-up angiographic findings in distal internal carotid artery (ICA) aneurysms treated by stent-assisted coil embolization using the Enterprise or Neuroform stent.
Materials and Methods
We included 68 patients with 70 aneurysms: 31 cases with Enterprise and 39 cases with Neuroform. Inclusion criteria were 1) location of the stent within the distal ICA, including the carotid siphon; 2) follow-up angiogram after > 6 months, and 3) single use of the stent for 1 parent artery.
Results
The patients' mean age was 54.9 years (16 male and 52 female). Mean follow-up duration was 9.1 months. At follow-up, there were intraluminal filling defects of the parent artery in 19.4% of the Enterprise group and no filling defect in the Neuroform group. There was no significant in-stent stenosis in either group. Straightening of the parent artery was seen in 35.5% of the Enterprise group and 20.5% of the Neuroform group. Two Enterprise cases showed delayed migration.
Conclusion
The Enterprise showed statistically significant intraluminal filling defects of the parent artery compared with the Neuroform. The rates of significant in-stent stenosis and straightening of the parent artery were not significantly different between the Enterprise and the Neuroform groups.
With the development of new endovascular techniques and devices, endovascular treatment for patients with intracranial aneurysms is increasingly being accepted as a safe and useful method (1, 2). However, wide-neck, large, and giant aneurysms are challenges in the conventional endovascular treatment of aneurysms (1, 3, 4, 5, 6). In these cases, stent placement is another treatment option and has been successfully used to treat intracranial aneurysms (1, 7). There are two types of self-expanding intracranial stents currently available. One has an open-cell design such as the Neuroform (Boston Scientific, Natick, MA, USA) and the other has a closed-cell design such as the Solitaire, Leo, and Enterprise (Cordis, Miami Lakes, FL, USA) (8). The Neuroform is an open-cell, nitinol, self-expanding microstent. The Enterprise is a closed-cell, nitinol, self-expanding, and retractable stent (9).
Stent deployment for a straight vessel is relatively simple, and there is no practical difference in using either stent. When deployed in a curved vessel, however, these two types of stents will result in different appearances of the parent artery and stent itself (10). The distal internal carotid artery (ICA), and especially the carotid siphon, is known to have a tortuous vasculature, as the name suggests. Thus stent deployment for the distal ICA including the carotid siphon involves a complicated anatomical region and might result in more complications during and after the stent deployment procedure.
Follow-up imaging after stent-assisted coil embolization (SACE) procedures has usually been focused on the presence or absence of residual filling in the aneurysm (7, 11). To our knowledge, no published studies have included an evaluation of the angiographic follow-up results focused on the distal ICA as the parent artery and comparison of the stent-deployment effects between these two stents in the SACE of distal ICA aneurysms.
In this study, we retrospectively analyzed and compared the follow-up angiographic results of distal ICA aneurysms including the carotid siphon, treated by a SACE technique using the Enterprise or the Neuroform. We discuss the stent-associated effects, focusing on the angiographic findings of the parent artery.
This study was approved by the institutional review board at our institution. Informed consent from patients was waived due to the retrospective study design.
Imaging data of distal ICA aneurysms treated with SACE from December 2008 to September 2012 were analyzed in this retrospective study. Inclusion criteria were: 1) the location of the stent within the distal ICA, including the paraclinoid ICA, the carotid siphon; 2) the availability of immediate post-implantation digital subtraction angiography (DSA) and follow-up DSA images from 6 months after the stent implantation, from February 2009 to April 2013; 3) the use of a self-expanding nitinol intracranial stent: the Enterprise or the Neuroform stent (4.5 mm in diameter by 28 mm in length for the Enterprise and 4.5 mm in diameter by 20 mm in length for the Neuroform); 4) single deployment of the stent for one parent artery (two or more stent insertions for the same side of the parent artery were excluded).
We reviewed our database for cases of aneurysms treated by SACE and found 68 patients with 70 aneurysms that met these criteria, including 31 cases with the Enterprise stent (Enterprise group) and 39 cases with the Neuroform stent (Neuroform group).
All endovascular procedures were performed using a biplane angiography unit with 3-dimensional rotational angiography capability (Axiom Artis Zee Biplane; Siemens, Erlangen, Germany). For patients with unruptured aneurysms, dual antiplatelet drugs [300 mg/day aspirin and 75 mg/day clopidogrel (Plavix; Sanofi-Synthelabo, Seoul, Korea)] were given for at least 3 days before the procedure. For patients with ruptured aneurysms, Tirofiban (Aggrastat, Merck & Co, Whitehouse Station, NJ, USA) was started after the aneurysms were secured with the coil.
In our institution, since June 2012 platelet inhibition function has been performed with the Multiplate® analyzer system (Dynabyte Medical, Munich, Germany) immediately before starting the procedure. If the patient was considered as a nonresponder [> 45 units (U) for the area under the curve of clopidogrel or > 30 U for that of aspirin], an additional anti-platelet drug was administered as soon as the procedure was finished.
All of the procedures were performed under local anesthesia. Endovascular access was obtained by a standard transfemoral approach unilaterally. After placement of the sheath, all patients received systemic heparinization: a 3000 to 5000 IU bolus of heparin (50 IU/kg body weight), followed by continuous infusion of 1000 to 2000 IU of heparin/h to double the baseline activated clotting time. A 6 Fr shuttle guiding catheter (Shuttle-SL; Cook, Bloomington, IN, USA) was placed into the proximal ICA. A stent delivery microcatheter (Prowler Select Plus, 2.3 Fr; Cordis, Miami Lakes, FL, USA, or Neurorenegade Hi-Flo, 2.8 Fr; Boston Scientific, Natick, MA, USA) was first navigated and positioned across the aneurysm neck. The stent was then loaded into the microcatheter and deployed by withdrawing the microcatheter while stabilizing the delivering wire. Three stenting strategies were employed in this study: the mesh technique, the jailing technique, and the salvaging stent placement (12). At the end of coiling, final angiographic images were acquired to confirm adequate coiling.
After the procedure, low-molecular-weight heparin (2850 IU/0.3 mL) (Fraxiparine; Sanofi-Synthelabo, Seoul, Korea) was administered subcutaneously for 2 days. All the patients were continued on aspirin and clopidogrel postoperatively for 6 months to 1 year, depending on the patient's condition.
All patients were followed up with DSA beyond 6 months after the initial SACE procedure. Angiograms were performed at high magnification in the working angles for the coil embolization and the stenting. Working angles were reproduced by optimizing the orientation of osseous landmarks with the stent markers and coil mass.
Follow-up DSA images were then compared with the immediate post-embolization angiograms with a similar projection and the same magnification ratio for the following parameters.
1) The term "intraluminal filling defect" was defined as a focal contrast filling defect at the stent-deployment area of the parent artery, between the stent filament and the vessel wall in the follow-up angiogram only (the "intraluminal filling defect" did not appear in the initial immediate post-embolization angiogram). And the presence of an incomplete intraluminal filling defect was recorded.
2) The stent-subtended arc angle, measured independently of the radius of the siphon curvature, was evaluated on the basis of the angle at which the stent was deployed in the parent vessel (7).
3) The carotid siphon angle was measured by the following method: first, the center was placed at the midpoint of the carotid siphon; second, a line was drawn between the center point and upper and lower portion of the carotid siphon; finally the angle between the two lines was measured.
4) The term "straightening" was defined as more than 10% of an angle increase of the parent artery between the immediate postembolization angiograms and the follow-up angiograms, using the stent-subtended arc angle and carotid siphon angle.
5) The mean diameter at the distal and proximal portion of the stent-deployment sites was measured to identify in-stent stenosis (or intimal hyperplasia). We defined the term "significant in-stent stenosis" as indicating more than 50% narrowing within the stent-deployment area of the parent artery.
6) Any changes in the stent itself on the follow-up angiogram, such as delayed stent migration, were also recorded.
7) Aneurysmal occlusion was graded on a 3-point Raymond scale (RS) (13): RS 1, complete obliteration of the aneurysm including the neck; RS 2, contrast filling of the neck of the aneurysm without opacification of the aneurysm sac; RS 3, contrast filling of the sac of the aneurysm.
Two radiologists evaluated all the images independently and these measurements and calculations were determined by consensus. All imaging data were reviewed on a Picture Archiving and Communication System (PACS) workstation (Maroview; version 5.4.10.52, Marotech, Seoul, Korea).
The patients' medical histories, procedural reports, and clinical outcomes were analyzed retrospectively by reviewing their electronic medical record charts. A complete neurologic examination was performed on all patients by a neurosurgeon, at the baseline, and immediately after the procedure and at the follow-up by experienced physicians certified in stroke assessment. A modified Rankin scale (mRS) score (14) was assessed at the follow-up evaluations. Any adverse events, especially ischemic stroke events, were checked and recorded.
All data were presented as mean ± standard deviation. Fisher's exact test was used for comparisons of categorical data. Student's t-test was used to determine statistical significance for continuous parameters including age, aneurysm size, stent-substented arc angle, carotid siphon angle, and significant in-stent stenosis. Significant differences were established for p < 0.05. Statistical analysis was performed by using MedCalc (version 12.7.0., MedCalc Software, Mariakerke, Belgium).
In total, 68 patients with 70 aneurysms were available for analysis. The patients' mean age was 54.9 ± 10.7 years (range, 26-78 years), including 16 men and 52 women. Patient demographic information and aneurysm characteristics are provided in Table 1.
Follow-up DSA images were performed for all patients, ranging from 6 to 36 months (mean 9.1 ± 4.7 months) after the initial stent-assisted coiling procedure. Most of the patients (66/68) had unilateral (right or left) stent insertion. However, 2 patients had bilateral Enterprise stent insertion for coiling of each side of distal ICA aneurysms.
The Enterprise group (n = 31) showed 6 (19.4%) cases of intraluminal filling defects at the stent-deployment area of the distal ICA and there was no filling defect of the distal ICA in the Neuroform group (Fig. 1). There was no significant in-stent stenosis in either group. One patient treated by SACE using the Neuroform was shown to have approximately 40% narrowing of the stent deployment area of the carotid siphon at the 6-month follow-up DSA (Fig. 2). Straightening of the parent artery was seen in 11/31 (35.5%) cases in the Enterprise group and 8/39 (20.5%) cases in the Neuroform group (Fig. 3). Among the 70 deployed stents, 2 Enterprise stents showed delayed migration on the follow-up images, but the length of migration was < 10 mm. Angiographic results are summarized in Table 2.
On the immediate post-treatment angiogram, the occlusion grades were as follows for the Enterprise group: RS 1 in 23 (74.2%), RS 2 in 4 (12.9%), and RS 3 in 4 (12.9%) aneurysms; in the Neuroform group, we observed RS 1 in 27 (69.2%), RS 2 in 8 (20.5%), and RS 3 in 4 (10.3%) aneurysms. The occlusion grades for the follow-up DSA in the Enterprise group were RS 1 in 21 (67.7%), RS 2 in 8 (25.8%), and RS 3 in 2 (6.5%); follow-up occlusion grades in the Neuroform group were RS 1 in 34 (87.2%), RS 2 in 4 (10.3%), and RS 3 in 1 (2.6%) aneurysms. Six (75%) of 8 aneurysms with persistent contrast filling of the sac were totally occluded
at the follow-up, whereas 2 (25%) showed persistent neck opacification. The frequency of aneurysms with RS 3 decreased from 8/70 (11.4%) on initial post-treatment angiogram evaluation to 3/70 (4.3%) on the follow-up angiogram, while the overall frequency of aneurysms with RS 1 increased slightly from 50/70 (71.4%) to 55/70 (78.6%). The initial and follow-up angiographic outcomes of aneurysms using the 3-point RS are summarized in Table 3.
In the Enterprise group with unruptured aneurysms (n = 26), none of the patients had neurologic symptoms at the baseline or at the follow-up. In the Enterprise group with ruptured aneurysms (n = 3), one patient (3.4%) showed mild lower extremity numbness (mRS = 1) at the follow-up. In the Neuroform group with unruptured aneurysms (n = 37), none of the patients except two had neurologic symptoms at the baseline. One patient (2.6%), who had been previously diagnosed with poliomyelitis, had left leg weakness (mRS = 2) at the baseline. Another patient (2.6%) initially had gait disturbance and weakness (mRS = 3) due to a previous cerebral infarction. At the follow-up, these two patients showed no additional neurologic symptoms. In the Neuroform group with ruptured aneurysms (n = 2), these two patients had severe headache and mental changes at the baseline but after treatment and at the follow-up, they showed no neurologic symptoms.
After the introduction of self-expandable flexible microstents, SACE has become a feasible option for the treatment of wide-neck aneurysms and more recently for small and medium-sized aneurysms (15, 16).
There are predominantly 2 types of self-expanding flexible microstents in use-the open-cell design Neuroform and the closed-cell design Enterprise. Although in vitro studies using angiographic flat-panel computed tomography (CT) have shown crumpling and ovalization of these stents during use of bending elastic tube models (10, 17), there are little in vivo data on the apposition of the intracranial stent-strut to the parent vessel wall, even after over 10 years of clinical use (17, 18). The siphon curvature of the ICA in humans has similar characteristics to the bending tube model. We analyzed the comparative follow-up angiographic findings of distal ICA aneurysms treated by SACE using the Enterprise and the Neuroform stent, focusing on the stent-subtended area. To our knowledge, however, there are no published in vivo reports focused on the distal ICA including the carotid siphon as the parent artery, using DSA for the follow-up evaluation.
The main findings of this comparative study are as follows: 1) an intraluminal filling defect of the parent artery in the Enterprise group (statistically significant difference), 2) no significant in-stent stenosis (statistically insignificant difference), 3) mild geometrical changes in the vasculature (statistically insignificant difference), and 4) delayed stent migration (statistically insignificant difference).
First, only the Enterprise group showed intraluminal filling defects of the parent artery (n = 6/31, 19.4%). Our results showed a statistically significant greater occurrence of intraluminal filling defects using the Enterprise compared with using the Neuroform (19.4% and 0%, respectively, p = 0.0056). We thought that incomplete stent apposition (ISA), defined as separation of one or more stent struts from the underlying vessel wall and observation of blood speckles between the stent struts and the vessel wall (19), and foreign body reaction around the struts (20) due to ISA were the main causes for the intraluminal filling defect. ISA in distal ICA aneurysms, with the stent-subtended area at the carotid siphon, may occur because the carotid siphon has a curved segment with a relatively large radius. In a recent study, Heller et al. (17) reported that for the ISA, the crescent sign as a distinctive semi-lunar signal pattern, identified using 3T magnetic resonance angiography (MRA), denoted flow outside the boundaries of the stent struts using the Enterprise stent. They reported that 17 of 18 cases with the crescent sign representing ISA were recognized when stents deposited in the comparatively tortuous ICA showed a probable link between the interaction with the architecture of the closed-cell design stent and the parent vessel anatomy. However, the number of recorded ISA in our study was relatively small compared with the study using 3T MRA within 3 days of the procedure. The lower incidence of ISA in our study might be due to the study design in which our results from the analysis of the DSA were taken from images obtained more than 6 months after the procedure. Because DSA is not a cross-sectional image and mainly shows intraluminal contrast filling of the vascular structure, ISA would be more clearly seen by MRA or flat-panel CT. In addition, different image analysis timings after the procedure may influence the detection of ISA.
A further study by Heller et al. (7) showed that the high prevalence of ISA in curved vessels may be a direct result of the inherited closed-cell design of the Enterprise stent. In larger diameter vessels, such as the distal ICA, the Enterprise stent showed a higher tendency for ISA, which might be due to the small outer radial force of the stent needed to deploy the vessel wall sufficiently. When the maximal diameter of the parent vessel is > 4 mm, stent dislodgement may occur (11). Therefore, deployment of the Enterprise in curved or tortuous vessels such as the carotid siphon with its larger diameter (> 4 mm) causes a greater incidence of ISA.
ISA may cause complications such as immediate flow reduction, embolism, and restenosis (21). In patients with coronary stent deployment, ISA can be associated with no neointimal hyperplasia. ISA without neointimal hyperplasia was significantly associated with the presence of thrombus at the follow-up, and may constitute a potent substrate for late stent thrombosis (22). There are several reports on the morphological changes, particularly conformity of the parent artery, after stent deployment (1, 7, 9, 10, 23, 24). In our study, the Enterprise group with ISA showed no additional neurologic symptoms at follow-up. To demonstrate the clinical significance of ISA in cases treated with Enterprise devices, further studies will be needed.
Second, after stent deployment, activation and proliferation of regional smooth muscle cells develops and may lead to neointimal hyperplasia, which can cause a re-stenosis within the stent (25, 26). Endothelial cells have a critical role in the control of smooth muscle growth, and when regulation is disturbed, neointimal hyperplasia causes stenosis (25, 27). In-stent stenosis is commonly encountered within 3-6 months after stent deployment (25, 28, 29). Some histopathologic reports have demonstrated that neointimal hyperplasia is implicated as the primary mechanism in in-stent stenosis (25, 30, 31). In our study, there was no significant in-stent stenosis in either of the two groups. There was no statistically significant difference between the two stents in the occurrence of significant in-stent stenosis (0% and 0%, respectively, p = 1). In our study, no patient showed any significant neurologic symptoms or sequelae. According to a recent review article using a computerized database search of SACE (32), the overall rate of delayed in-stent stenosis was 5.3%, with individual study rates varying from 0% to 20.6%, which were favorable outcomes compared with our result.
Third, based on our literature review, significant changes in the geometry of the native intracranial vasculature as a result of stent deployment were confirmed by several previous studies (1, 7, 23). Our study also showed alteration of the carotid siphon vasculature, demonstrated as "straightening", after stent implantation. Stent placement significantly changes the parent artery-aneurysm angle and also the angle between the afferent and efferent vessels. This may perform an important role in the alteration of the local hemodynamics, encouraging the healing of aneurysms (1). In our study, the occurrence of straightening between the Enterprise and the Neuroform was not significantly different (35.5% and 20.5%, respectively, p = 0.1856).
Fourth, there have been reports of delayed migration related to the Enterprise stent (9, 33, 34, 35). According to the literature, possible explanations for this delayed migration include the inherited structural characteristics of the Enterprise, the diameter difference between either side of the stent-deployed vessels, and the angle formed between the locations proximal and distal to the stent expanding after stent placement. Similarly to a previous study (9), we found the phenomenon of angle expansion, defined as straightening in our study, associated with the use of not only the Enterprise stent but also the Neuroform stent. After angular expansion, the stented segment of the distal portion may develop closer in line with the proximal portion, thus decreasing resistance of stent migration proximally. In our study, delayed migrations were seen only with the Enterprise stent.
Our initial angiographic results showed that 62/70 (88.6%) of aneurysms treated with SACE had total occlusion (RS 1) or only minimal flow into the neck (RS 2) (Table 3), and follow-up angiograms showed 67/70 (95.7%) of aneurysms had no flow or only minimal flow into the neck of the aneurysms. Consistent with previous reports (11, 18, 36, 37, 38), initial subtotal occlusion tends to progress to total occlusion in either type of stent.
There were several limitations in this study. First, the study was retrospective in nature with the possibility of selection bias. Second, the number of patients was relatively small. Further studies are required with a larger number of cases and adequate follow-up to identify whether the long-term outcome of endovascular treatment is affected by vascular configuration change. Third, in this study, the measurements were acquired from selected 2-dimensional projection angiographic images using the PACS workstation. As mentioned by the authors of a previous study (1, 23), it is very difficult to describe the morphologic changes in 3-dimensional vascular geometry by using 3-dimensional angiographic datasets and to determine uniform measurement standards. We therefore used the same working view angle to reduce error.
In conclusion, our study is a mid-term comparative follow-up of stent-assisted coil embolization with two stents (the Enterprise and the Neuroform) in distal ICA aneurysms including aneurysms of the carotid siphon. With regard to the parent artery, the Enterprise may cause statistically significant intraluminal filling defects in the carotid siphon compared with the Neuroform. The rates of significant in-stent stenosis or straightening of the parent artery were not statistically different between the Enterprise and the Neuroform.
Figures and Tables
Fig. 1
Intraluminal filling defect (incomplete stent apposition).
A. A 67-year-old woman has a left P-com aneurysm (arrowhead) and two right paraclinoid aneurysms (arrow and open arrow). Immediate post-procedural angiography shows right paraclinoid ICA aneurysm after stent-assisted coiling using a 4.5 × 28 mm Enterprise stent with complete occlusion and a just proximal paraclinoid aneurysm treated by stent only.
B. Eight-month follow-up angiography reveals an intraluminal filling defect (arrow) at the substented area of the siphon (between proximal arrowhead and distal arrowhead).
Note.-ICA = internal carotid artery
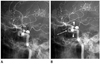
Fig. 2
In-stent stenosis. A 64-year-old woman with an incidentally found aneurysm.
A. Immediate post-procedural angiography shows left paraclinoid ICA aneurysm after stent-assisted coiling using a 4.5 × 20 mm Neuroform stent. Subtle contrast filling of the aneurysm sac is seen.
B. Six-month follow-up angiography shows that the aneurysm underwent progressive occlusion as there is no residual flow visible. In addition, there is mild (40%) narrowing of substented area of the parent artery. But it is not significant in-stent stenosis, according to our definition: we defined the term "significant in-stent stenosis" as more than 50% narrowing within the stent-deployment area of the parent artery.
Note.-ICA = internal carotid artery
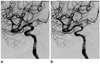
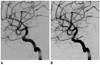
Fig. 3
Parent artery straightening. A 36-year-old woman presented with severe headache. Examination revealed subarachnoid hemorrhage due to ruptured aneurysm.
A. Immediate post-procedural angiography shows a right paraclinoid ICA aneurysm after stent-assisted coiling using a 4.5 × 28 mm Enterprise stent with complete occlusion.
B. Twelve-month follow-up angiography reveals increased vascular curvature of the carotid siphon ("straightening") and focal intraluminal filling defect at the substented area (arrow).
Note.-ICA = internal carotid artery
References
1. Huang QH, Wu YF, Xu Y, Hong B, Zhang L, Liu JM. Vascular geometry change because of endovascular stent placement for anterior communicating artery aneurysms. AJNR Am J Neuroradiol. 2011; 32:1721–1725.
2. Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol. 2009; 8:427–433.
3. Wakhloo AK, Linfante I, Silva CF, Samaniego EA, Dabus G, Etezadi V, et al. Closed-cell stent for coil embolization of intracranial aneurysms: clinical and angiographic results. AJNR Am J Neuroradiol. 2012; 33:1651–1656.
4. Lavine SD, Larsen DW, Giannotta SL, Teitelbaum GP. Parent vessel Guglielmi detachable coil herniation during wide-necked aneurysm embolization: treatment with intracranial stent placement: two technical case reports. Neurosurgery. 2000; 46:1013–1017.
5. Phatouros CC, Sasaki TY, Higashida RT, Malek AM, Meyers PM, Dowd CF, et al. Stent-supported coil embolization: the treatment of fusiform and wide-neck aneurysms and pseudoaneurysms. Neurosurgery. 2000; 47:107–113. discussion 113-115.
6. Gallas S, Pasco A, Cottier JP, Gabrillargues J, Drouineau J, Cognard C, et al. A multicenter study of 705 ruptured in tracranial aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol. 2005; 26:1723–1731.
7. Heller RS, Malek AM. Parent vessel size and curvature strongly influence risk of incomplete stent apposition in enterprise intracranial aneurysm stent coiling. AJNR Am J Neuroradiol. 2011; 32:1714–1720.
8. Krischek O, Miloslavski E, Fischer S, Shrivastava S, Henkes H. A comparison of functional and physical properties of self-expanding intracranial stents [Neuroform3, Wingspan, Solitaire, Leo+, Enterprise]. Minim Invasive Neurosurg. 2011; 54:21–28.
9. Gao B, Malek AM. Possible mechanisms for delayed migration of the closed cell--designed enterprise stent when used in the adjunctive treatment of a basilar artery aneurysm. AJNR Am J Neuroradiol. 2010; 31:E85–E86.
10. Ebrahimi N, Claus B, Lee CY, Biondi A, Benndorf G. Stent conformity in curved vascular models with simulated aneurysm necks using flat-panel CT: an in vitro study. AJNR Am J Neuroradiol. 2007; 28:823–829.
11. Lubicz B, François O, Levivier M, Brotchi J, Balériaux D. Preliminary experience with the enterprise stent for endovascular treatment of complex intracranial aneurysms: potential advantages and limiting characteristics. Neurosurgery. 2008; 62:1063–1069. discussion 1069-1070.
12. Yang PF, Liu JM, Huang QH, Zhao WY, Hong B, Xu Y, et al. Preliminary experience and short-term follow-up results of treatment of wide-necked or fusiform cerebral aneurysms with a self-expanding, closed-cell, retractable stent. J Clin Neurosci. 2010; 17:837–841.
13. Raymond J, Roy D, Bojanowski M, Moumdjian R, L'Espérance G. Endovascular treatment of acutely ruptured and unruptured aneurysms of the basilar bifurcation. J Neurosurg. 1997; 86:211–219.
14. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988; 19:604–607.
15. Maldonado IL, Machi P, Costalat V, Mura T, Bonafé A. Neuroform stent-assisted coiling of unruptured intracranial aneurysms: short- and midterm results from a single-center experience with 68 patients. AJNR Am J Neuroradiol. 2011; 32:131–136.
16. Turk AS, Niemann DB, Ahmed A, Aagaard-Kienitz B. Use of self-expanding stents in distal small cerebral vessels. AJNR Am J Neuroradiol. 2007; 28:533–536.
17. Heller RS, Miele WR, Do-Dai DD, Malek AM. Crescent sign on magnetic resonance angiography revealing incomplete stent apposition: correlation with diffusion-weighted changes in stent-mediated coil embolization of aneurysms. J Neurosurg. 2011; 115:624–632.
18. Piotin M, Blanc R, Spelle L, Mounayer C, Piantino R, Schmidt PJ, et al. Stent-assisted coiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive aneurysms. Stroke. 2010; 41:110–115.
19. Rathore S, Terashima M, Habara M, Kinoshita Y, Nasu K, Katoh O, et al. Incomplete stent apposition after coronary stent implantation: myth or reality? J Interv Cardiol. 2009; 22:341–349.
20. Bennett MR. Vascular pathology as a result of drug-eluting stents. Heart. 2007; 93:895–896.
21. du Mesnil de Rochemont R, Yan B, Zanella FE, Rüfenacht DA, Berkefeld J. Conformability of balloon-expandable stents to the carotid siphon: an in vitro study. AJNR Am J Neuroradiol. 2006; 27:324–326.
22. Ozaki Y, Okumura M, Ismail TF, Naruse H, Hattori K, Kan S, et al. The fate of incomplete stent apposition with drug-eluting stents: an optical coherence tomography-based natural history study. Eur Heart J. 2010; 31:1470–1476.
23. King RM, Chueh JY, vanderBom IM, Silva CF, Carniato SL, Spilberg G, et al. The effect of intracranial stent implantation on the curvature of the cerebrovasculature. AJNR Am J Neuroradiol. 2012; 33:1657–1662.
24. Gao B, Baharoglu MI, Cohen AD, Malek AM. Stent-assisted coiling of intracranial bifurcation aneurysms leads to immediate and delayed intracranial vascular angle remodeling. AJNR Am J Neuroradiol. 2012; 33:649–654.
25. Yoon KW, Kim YJ. In-stent stenosis of stent assisted endovascular treatment on intracranial complex aneurysms. J Korean Neurosurg Soc. 2010; 48:485–489.
26. Kipshidze N, Dangas G, Tsapenko M, Moses J, Leon MB, Kutryk M, et al. Role of the endothelium in modulating neointimal formation: vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. J Am Coll Cardiol. 2004; 44:733–739.
27. Wakhloo AK, Mandell J, Gounis MJ, Brooks C, Linfante I, Winer J, et al. Stent-assisted reconstructive endovascular repair of cranial fusiform atherosclerotic and dissecting aneurysms: long-term clinical and angiographic follow-up. Stroke. 2008; 39:3288–3296.
28. Marks MP, Wojak JC, Al-Ali F, Jayaraman M, Marcellus ML, Connors JJ, et al. Angioplasty for symptomatic intracranial stenosis: clinical outcome. Stroke. 2006; 37:1016–1020.
29. Fiorella D, Albuquerque FC, Woo H, Rasmussen PA, Masaryk TJ, McDougall CG. Neuroform in-stent stenosis: incidence, natural history, and treatment strategies. Neurosurgery. 2006; 59:34–42. discussion 34-42.
30. Kearney M, Pieczek A, Haley L, Losordo DW, Andres V, Schainfeld R, et al. Histopathology of in-stent restenosis in patients with peripheral artery disease. Circulation. 1997; 95:1998–2002.
31. Hoffmann R, Mintz GS, Dussaillant GR, Popma JJ, Pichard AD, Satler LF, et al. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation. 1996; 94:1247–1254.
32. McLaughlin N, McArthur DL, Martin NA. Use of stent-assisted coil embolization for the treatment of wide-necked aneurysms: a systematic review. Surg Neurol Int. 2013; 4:43.
33. Rodriguez GJ, Maud A, Taylor RA. Another delayed migration of an enterprise stent. AJNR Am J Neuroradiol. 2009; 30:E57.
34. Kelly ME, Turner RD 4th, Moskowitz SI, Gonugunta V, Hussain MS, Fiorella D. Delayed migration of a self-expanding intracranial microstent. AJNR Am J Neuroradiol. 2008; 29:1959–1960.
35. Lavine SD, Meyers PM, Connolly ES, Solomon RS. Spontaneous delayed proximal migration of enterprise stent after staged treatment of wide-necked basilar aneurysm: technical case report. Neurosurgery. 2009; 64:E1012. discussion E1012.
36. Izar B, Rai A, Raghuram K, Rotruck J, Carpenter J. Comparison of devices used for stent-assisted coiling of intracranial aneurysms. PLoS One. 2011; 6:e24875.
37. Sedat J, Chau Y, Mondot L, Vargas J, Szapiro J, Lonjon M. Endovascular occlusion of intracranial wide-necked aneurysms with stenting (Neuroform) and coiling: mid-term and long-term results. Neuroradiology. 2009; 51:401–409.
38. Biondi A, Janardhan V, Katz JM, Salvaggio K, Riina HA, Gobin YP. Neuroform stent-assisted coil embolization of wide-neck intracranial aneurysms: strategies in stent deployment and midterm follow-up. Neurosurgery. 2007; 61:460–468. discussion 468-469.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download