Abstract
Objective
To preliminarily evaluate the diagnostic performance of an unenhanced MRI for detecting hepatocellular carcinoma (HCC) with a case-control study design.
Materials and Methods
The case group consisted of 175 patients with initially-diagnosed HCC, who underwent a 3T liver MRI. A total of 237 HCCs were identified. The number of HCCs that were smaller than 1 cm, 1 cm ≤ and < 2 cm, and ≥ 2 cm were 19, 105, and 113, respectively. For the control group, 72 patients with chronic liver disease, who did not have HCC, were enrolled. Two radiologists independently reviewed the T2 half-Fourier acquisition single-shot turbo spin echo, T2 fast spin echos with fat saturation, T1 gradient in- and out-of-phase images, and diffusion-weighted images/apparent diffusion coefficient maps to detect HCC. Per-patient analyses were performed to evaluate the sensitivity and specificity of the non-contrast MRI for diagnosing HCC. Furthermore, the per-lesion sensitivity was also calculated according to tumor size.
Results
In the per-patient analyses, the sensitivity and specificity of reader 1 were 86.3% (151/175) and 87.5% (63/72), respectively; while those of reader 2 were 82.9% (145/175) and 76.4% (55/72), respectively. When excluding HCCs smaller than 1 cm, the sensitivity of reader 1 and 2 were 88.0% (147/167) and 86.2% (144/167), respectively. In the per-lesion analyses, the sensitivities of reader 1 and reader 2 were 75.9% (180/237) and 70.5% (167/237), respectively.
Most hepatocellular carcinomas (HCC) occur in patients with a known risk factor. The most common risk factor of HCC worldwide is liver cirrhosis that is caused by viral hepatitis, followed by alcoholic liver disease, non-alcoholic fatty liver disease, genetic hemochromatosis, and advanced stage primary biliary cirrhosis (12).
More than 70% of HCC patients are diagnosed with advanced stage cancer that is not amenable to potential curative therapy (3). Therefore, the most effective ways to reduce the mortality of HCC are preventing/treating hepatitis viral infection and early diagnosis of HCC through regular surveillance. The prospective surveillance of patients at high risk of developing HCC increases the proportion diagnosed with potentially curable disease and improves HCC-related mortality (45).
The European Association for the Study of the Liver (EASL), the American Association for Study of Liver Diseases (AASLD), and Asian Pacific Association for the Study of the Liver recommend biannual ultrasonography (US) for patients with risk factors for the surveillance of HCC (4678). However, the sensitivity of an US for HCC detection is not satisfactory. In a systematic review, the sensitivity of US for detecting early HCC, which means HCC is amenable to curative treatment, was only 63% (9). Therefore, other imaging tools are being explored as alternatives for HCC surveillance.
Recently, Kim et al. (10) compared the diagnostic performance of non-contrast MRIs, including diffusion weighted imaging (DWI), to contrast-enhanced MRIs for detecting liver malignancy in patients with chronic liver disease. In their study, the sensitivity and specificity of the contrast-enhanced and non-contrast MRI sets for detecting liver malignancies and distinguishing them from benign lesions were comparable. This study implies that non-contrast MRIs, which is relatively cheap, safe, and easy to perform compared to contrast enhance CTs or MRIs, could be a candidate HCC surveillance tool.
Therefore, in this retrospective study, the diagnostic performance of a non-contrast liver MRI to detect HCCs was evaluated with a case-control study design.
This study was performed with the approval of the Institutional Review Board of our institution, and informed consent was waived due to the retrospective nature of this study.
The inclusion criteria for this study were as follows: 1) initially diagnosed HCC patients with no previous treatment history, 2) patients within the Milan criteria (i.e., [a] one tumor smaller than 5 cm, [b] up to 3 tumors smaller than 3 cm, and [c] no macrovascular invasion or extrahepatic metastases), because they were candidates for curative treatment and allowed the comparison of this study's data to those of a meta-analysis by Singal et al. (9).
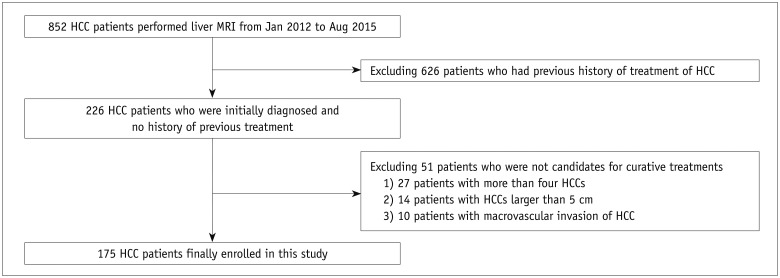
From January 2012 to August 2015, a total of 226 HCC patients initially diagnosed with HCC and with no prior treatment history underwent a liver MRI at our institute. We excluded 51 patients because: 1) they had more than four HCCs (n = 27); 2) an HCC ≥ 5.0 cm (n = 14); or 3) macrovascular invasion (n = 10). Finally, 175 patients (male:female = 137:38; mean age, 62.5 ± 10.1 years) were enrolled in this study as the patient group (Fig. 1). HCC was diagnosed by surgical resection or liver transplantation in 37 patients and biopsy in 5 patients. In the remaining patients, the diagnosis of HCC was based on the radiological hallmarks of AASLD, or the Korean Liver Cancer Study Group-National Cancer Center Korea (KLCSG-NCC), namely the arterial enhancement and washout on the portal venous or transitional phases (861112). HCCs smaller than 1 cm were diagnosed based on the imaging findings in the same manner according to the KLCSG-NCC guidelines (11). The low signal intensity of the hepatobiliary phase as washout was not considered because the hypointensity of the hepatobiliary phase is largely influenced by the uptake of the hepatocyte-specific contrast agent by hepatocytes (13).
A total of 237 HCCs were identified among the 175 patients in the patient group. One hundred twenty-nine patients (73.7%) had a single HCC, 30 patients (17.1%) had two HCCs, and 16 patients (9.1%) had three HCCs. Of the 237 HCCs, 19 (8.0%) were less than 1 cm, 105 HCCs (44.3%) were 1 cm ≤ and < 2 cm, and 113 (47.7%) were larger than 2 cm. Eight patients only had HCCs smaller than 1 cm (8/175, 4.6%). All HCCs less than 1 cm were pathologically confirmed by hepatic resection or transplantation.
As the control group, 72 chronic liver disease patients (male:female = 48:24; mean age, 57.5 ± 8.2 years) who did not have HCC and underwent a liver MRI in the same period, were enrolled. The non-existence of HCC in control patients was also confirmed with a follow-up dynamic CT or MRI (minimum follow-up period, 12 months).
The presence of underlying liver disease and liver cirrhosis were also evaluated in the patient and control groups. Liver cirrhosis was diagnosed by magnetic resonance (MR) imaging with the typical findings of 1) surface nodularity, 2) shrinkage of the liver parenchyma with hypertrophy of the left hemiliver or caudate lobe, and 3) presence of splenomegaly or varix. There was no statistically significant difference between the patient and control groups for the incidence of cirrhosis (p = 0.122, by the chi-square test). The characteristics of the study population are summarized in Table 1.
All liver MRIs were performed with a 3T system (Verio, Siemens, Erlangen, Germany) with an 8-channel phased-array torso coil. Breath-hold Half-Fourier Acquisition Single-shot Turbo spin Echo, respiratory triggered fast spin echo T2-weighted image (T2WI) with fat suppression and dual-gradient echo T1-weighted image (T1WI) using in-phase and opposed-phase were obtained. DWI with echo planar imaging using b-values of 0 s/mm2 and 500 s/mm2 were performed. An apparent diffusion coefficient (ADC) map was synthesized using all DWI.
For contrast enhancement, 0.025 mmol/kg gadoxetic acid (Eovist or Primovist, Bayer Healthcare, Berlin, Germany) was injected at a rate of 2 mL/s by an automated infusion system and followed by a 20-mL saline chaser. Three-dimensional gradient-recalled echo images were obtained in precontrast, arterial (with bolus tracking technique), portal venous (65- to 80-second delay), transitional (180-second delay), and hepatobiliary phases (20-minute delay).
The sum of the acquisition times for non-contrast liver MRIs were 8–10 minutes. MRI sequence parameters are summarized in Table 2.
Magnetic resonance images were retrospectively and independently evaluated by two abdominal radiologists with 19 years and 12 years of experience in liver imaging, respectively. Randomly distributed patient and control group images were given to both radiologists, and they were blinded to the contrast enhanced images and were unaware of the presence or location of any liver lesions or of the results of the imaging findings. Only non-contrast images were provided and MRI findings favoring HCC on the non-contrast MRI were: 1) high signal intensity lesions on T2WI, except for bright signal intensity implying cysts or hemangiomas; 2) fat components on T1WI in- and out-of-phase; and 3) diffusion restriction of DWIs, i.e., high signal intensity on DWI with low signal intensity on the ADC map (Fig. 2). If any two of these findings favoring HCC were noted in the focal hepatic lesions, readers considered those lesions as “suspicious lesions” that required an additional contrast-enhanced CT or MRI for the recall process and recorded the location and size of these suspicious lesions. We adopted DWI with b = 500 s/mm2 instead of b = 800 s/mm2 because 1) higher b-value images displayed more prominent distortion in some cases and 2) the purpose of this study was to evaluate the possibility of a non-contrast MRI as a surveillance tool, and higher b-value images, such as 800 s/mm2, can be performed only with the high-level MRI units, which may not be available in a surveillance setting.
After independent review, two reviewers re-evaluated the MR findings of each HCC in consensus in terms of the presence of findings that favored HCC to clarify the incidence of these findings.
Per-patient and per-lesion analyses were performed to assess the diagnostic performance of non-contrast MRIs for detecting HCCs. For per-patient analyses, the sensitivity and specificity for detecting HCC patients were calculated for both readers. Per-patient analyses were also conducted by excluding HCC patients who only had HCCs less than 1 cm because nodules or lesions smaller than 1 cm are not considered HCCs in the AASLD or EASL guidelines for US surveillance. The causes of false positive and false negative results were also analyzed by reviewing the MR images. The Kappa coefficient values were calculated to determine the inter-reader agreement for the per-patient analysis.
Per-lesion analyses were performed to calculate the sensitivity for each HCC. Sensitivities were calculated for all HCCs: HCCs ≥ 2 cm, HCCs ≥ 1 cm and < 2 cm, and HCCs < 1 cm in diameter.
The per-patient sensitivities of the two readers for all HCC patients and those HCC patients with HCCs ≥ 1 cm were also compared using the Fisher's exact test. For all statistical tests, a p value of < 0.05 was considered a statistically significant difference. All statistical analyses were performed using commercially available software (Medcalc, Medcalc software, Ostend, Belgium).
In a consensus review, a high signal intensity on T2WI was found in 81% (192/237) of all HCCs. The incidence of a T2WI high signal intensity varied according to the tumor size. The incidences of T2WI high signal intensities of HCC < 1 cm, 1 cm ≤ and < 2 cm, and ≥ 2 cm were 42.1% (8/19), 76.2% (80/105), and 92.0% (104/113), respectively. Diffusion restriction was detected in 78.5% (186/237) of HCCs and the incidences of diffusion restrictions of HCC < 1 cm, 1 cm ≤ and < 2 cm, and ≥ 2 cm were 47.4% (9/19), 72.4% (76/105) and 90.3% (102/113), respectively. A fat signal was found in only three patients (1.3%).
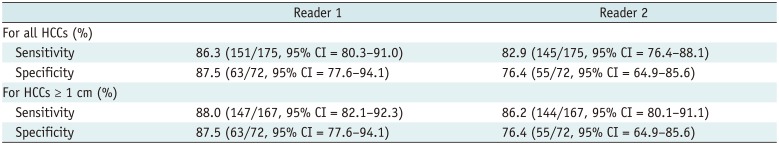
The sensitivity for detecting HCC patients was 86.3% and 82.9% for readers 1 and 2, respectively. The specificity was 87.5% for reader 1 and 76.4% for reader 2. The sensitivities for patients with HCCs ≥ 1 cm was 88.0% for reader 1 and 86.2% for reader 2. There were no significant differences in the sensitivities for all HCCs patients versus patients with HCCs ≥ 1 cm; the p values for readers 1 and 2 were 0.747 and 0.455, respectively. The sensitivities of readers 1 and 2 for patients who had only HCCs smaller than 1 cm were 62.5% (5/8) and 12.5% (1/8), respectively. The Kappa coefficient value for per-patient analyses for the two readers was 0.668, which indicates substantial agreement. The results of the per-patient analyses are summarized in Table 3.
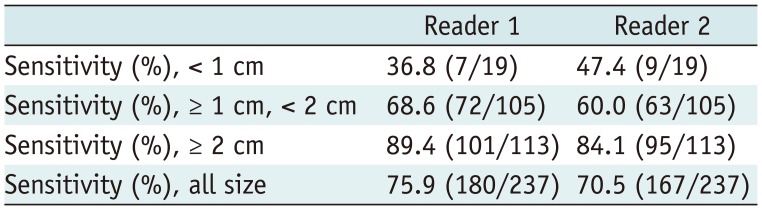
The sensitivities of readers 1 and 2 for all HCCs were 75.9% and 70.5%, respectively. In a subgroup analysis by tumor size, the sensitivities decreased with a decrease in the size of HCCs, and the sensitivities for HCCs that were < 1 cm were less than 50% for both readers. For HCCs larger than 2 cm, the sensitivities were above 80% for both readers. The results of per-lesion analyses are summarized in Table 4.
Eighteen HCCs in 16 patients were not detected by either reader. When reviewed retrospectively, these HCCs were not apparent in the non-contrast MRIs in 11 patients (Fig. 3), depicted as faintly hyperintense lesions only on DWI (n = 2), and visualized as too high of signal intensity on T2WI and misdiagnosed as a cyst (n = 1). Furthermore, one misdiagnosis was due to missing fat density (n = 1); while another was due to poor visualization of the tumor because of its location on the liver surface (n = 1). In two patients with visualization on DWI only, there were some artifacts on the DWI, and the detection of the tumor was very difficult due to poor image quality.
Eight control patients were diagnosed as HCC patients by both readers. When these cases were reviewed retrospectively, the focal lesions in four patients were hemangiomas (Fig. 4). Two patients had focal eosinophilic necrosis; while one patient had a focal nodular hyperplasia and one patient had a dysplastic nodule without arterial enhancement. For reader 1, the additional cause of false positive was a hepatic cyst (n = 1). Reader 2 additionally misdiagnosed hemangiomas (n = 2), hepatic abscess (n = 2), a focal nodular hyperplasia (n = 1), a dysplastic nodule (n = 1), a hepatic cyst (n = 1) and prominent vascular structures (n = 2).
In this study, the per-patient sensitivity for HCCs with a diameter of less than 5 cm was 82.9–86.3%, which is much better than the sensitivity of US in previous meta-analyses (914). The per-patient sensitivity did not significantly improve when patients who only had HCCs smaller than 1 cm were excluded, most likely because only 4.6% of all patients in our study had HCCs smaller than 1 cm. The per-lesion sensitivity for all HCCs was 70.5–75.9%, which is lower than that of the per-patient sensitivity. However, the per-lesion sensitivity for HCCs smaller than 1 cm was only 36.8–47.4%. Yu et al. (15) reported that the sensitivity for HCCs ≤ 1 cm was 46.0% with gadoxetic acid enhancement. Their results are similar to those of this study; in their study, the hyperintensity on T2WI and diffusion restriction on DWI were observed in 43% and 28% of HCCs smaller than 1 cm, respectively. The AASLD and EASL recommend not performing an additional recall process for hepatic nodules smaller than 1 cm found on a surveillance US; therefore, the low detection sensitivity for HCCs smaller than 1 cm is not a drawback of non-contrast MRIs as a surveillance tool for HCC when compared to US surveillance.
The per-patient specificity of our study is poorer than the values reported in previous studies (914). However, the higher specificities reported in previous studies were at the cost of lower sensitivities. Considering the importance of the early detection of HCC for better treatment outcomes, a premium is placed on sensitivity. The specificity of a surveillance tool is directly correlated to the false-referral rate, which can be calculated in a real surveillance setting.
Because of disappointing US surveillance results, many investigators have been researching better tools for HCC surveillance. A contrast-enhanced CT or MRI is considered an alternative option to US for HCC surveillance (461416). The Japanese Society of Hepatology recommend dynamic CT/MRI or gadoxetic acid-enhanced MRI for patients in the very-high HCC risk group, i.e., those with liver cirrhosis related to hepatitis B or C (17). However, a multiphasic CT or MRI using contrast media is relatively inadequate for repeated surveillance because of the associated patient inconvenience, high cost, side effects of contrast media, and repeated radiation exposure in the case of a CT (18). Pocha et al. (19) reported the results of a randomized, prospective study comparing the performance of US and multiphasic CT for HCC surveillance. In their study, biannual US was marginally more sensitive and less costly than annual CT. However, in that study, the sample size was small, and there were numerous off-protocol patients (approximately one-third of HCC patients).
Improved MRI performance due to multichannel surface receiver coils, parallel imaging techniques, fat suppression schemes, DWI, adoption of the hepatocyte specific contrast agents and multiple arterial phases has improved the performance of liver MRI for lesion detection and liver function evaluation (2021222324252627). However, as mentioned above, an enhanced MRI has some limitations and the focus of this study was on a non-contrast MRI as a potential HCC surveillance tool. Compared to an enhanced CT or MRI, a non-contrast MRI is easy to perform, requires less acquisition time, has no need for contrast media (i.e., lower costs and no side effects compared to enhanced MRI), has no associated radiation hazard, and is relatively inexpensive. In this study, the total examination time was about 10 minutes only. A previous study performed in the late 2000s reported modest sensitivity (52–55%) and high specificity (88–90%) of non-contrast MRIs compared to contrast-enhanced MRIs (sensitivity, 81–84%; specificity, 62–65%) in liver transplantation patients (28). However, according to Kim et al. (10) in 2013, the sensitivity (91.2–95.1%) and specificity (66.7–79.4%) of non-contrast MRIs are comparable to those of contrast-enhanced MRI (sensitivity, 93.9–95.9%; specificity, 75.7–82.4%). This study's results are similar to those of Kim and colleagues. However, the study by Kim et al. (10) only enrolled pathologically confirmed patients; while this study enrolled both pathologically confirmed and image-based diagnosed HCC patients. The imaging based diagnosis of HCC is already widely adopted clinically. Therefore, the results of this study reflect the real-world situation.
The most important obstacle for using a non-contrast MRI for the surveillance of HCC may be the higher cost of MRI examinations. A few studies have compared the cost-effectiveness of contrast-enhanced CTs or MRIs to US for HCC surveillance (293031). Andersson et al. (31) reported that the cost-effectiveness of a biannual US was better than that of an annual CT or MRI. However, in their study, the sensitivity of the US was 75%, which is higher than that reported in other studies. Furthermore, the cost of a MRI in that study was very high (four-fold higher than US), because the MRI was based on contrast-enhanced examinations. In Korea, the national medical insurance fee is about 100 US$ for a liver US and 250 US$ for a non-contrast liver MRI, which is much cheaper than the United States.
The annual incidence of HCC in targeted patients is another key factor of the cost-effectiveness (323334). Sarasin et al. (33) reported that the cost-effectiveness was much better for patients at higher risk of HCC development. Therefore, if the focus is on patients at higher risk of HCC development, the higher costs of MRI can be justified.
This study had several limitations. First, it was a retrospective case-control study and not performed in a surveillance setting, even though there was an attempt to simulate it. The prevalence of HCC is quite lower than that of this study in the real surveillance setting and selection bias might have been present in this study. Therefore, some statistical factors such as positive/negative predictive value could not be evaluated because these statistics are influenced by the prevalence of HCC. However, selecting initially diagnosed patients and adding a control group helped to simulate the surveillance setting and to evaluate the performance of non-contrast MRIs indirectly. With this limitation, the results of this study may not be generalizable. However, the results of this study may provide conceptual proof about the feasibility of using non-contrast MRIs for HCC surveillance. Second, the performance of non-contrast MRIs were not compared with those of other imaging methods such as US or contrast-enhanced MRI. In fact, a contrast-enhanced MRI was used as the reference standard, which might have overestimated the performance of non-contrast MRI. Finally, this study involved single institution data from a hepatitis B virus endemic area. The inclusion of patients with cirrhosis due to other causes might have changed the findings.
In conclusion, in this case-control feasibility study, the per-patient sensitivity and specificity of non-contrast MRI for detecting HCC were within reasonable ranges. Non-contrast MRI may have potential for the surveillance of HCC. Prospective diagnostic test accuracy studies using proper consecutive clinical cohorts are needed for further confirm the preliminary results of this study.
References
1. Lodato F, Mazzella G, Festi D, Azzaroli F, Colecchia A, Roda E. Hepatocellular carcinoma prevention: a worldwide emergence between the opulence of developed countries and the economic constraints of developing nations. World J Gastroenterol. 2006; 12:7239–7249. PMID: 17143937.

2. Tanaka M, Katayama F, Kato H, Tanaka H, Wang J, Qiao YL, et al. Hepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measures. J Epidemiol. 2011; 21:401–416. PMID: 22041528.

3. Meissner HI, Smith RA, Rimer BK, Wilson KM, Rakowski W, Vernon SW, et al. Promoting cancer screening: learning from experience. Cancer. 2004; 101(5 Suppl):1107–1117. PMID: 15316913.

4. Tan CH, Low SC, Thng CH. APASL and AASLD consensus guidelines on imaging diagnosis of hepatocellular carcinoma: a review. Int J Hepatol. 2011; 2011:519783. PMID: 22007313.

5. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004; 130:417–422. PMID: 15042359.

6. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022. PMID: 21374666.

7. Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010; 4:439–474. PMID: 20827404.

8. European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56:908–943. PMID: 22424438.
9. Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009; 30:37–47. PMID: 19392863.

10. Kim YK, Kim YK, Park HJ, Park MJ, Lee WJ, Choi D. Noncontrast MRI with diffusion-weighted imaging as the sole imaging modality for detecting liver malignancy in patients with high risk for hepatocellular carcinoma. Magn Reson Imaging. 2014; 32:610–618. PMID: 24702980.

11. Yoon JH, Park JW, Lee JM. Noninvasive diagnosis of hepatocellular carcinoma: elaboration on Korean Liver Cancer Study Group-National Cancer Center Korea practice guidelines compared with other guidelines and remaining issues. Korean J Radiol. 2016; 17:7–24. PMID: 26798212.

12. Korean Society of Abdominal Radiology. Diagnosis of hepatocellular carcinoma with gadoxetic acid-enhanced MRI: 2016 consensus recommendations of the Korean Society of Abdominal Radiology. Korean J Radiol. 2017; 18:427–443. PMID: 28458595.
13. Joo I, Lee JM, Lee DH, Jeon JH, Han JK, Choi BI. Noninvasive diagnosis of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout? Eur Radiol. 2015; 25:2859–2868. PMID: 25773941.

14. Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006; 101:513–523. PMID: 16542288.
15. Yu MH, Kim JH, Yoon JH, Kim HC, Chung JW, Han JK, et al. Small (≤1-cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology. 2014; 271:748–760. PMID: 24588677.

16. Davarpanah AH, Weinreb JC. The role of imaging in hepatocellular carcinoma: the present and future. J Clin Gastroenterol. 2013; 47(Suppl):S7–S10. PMID: 23632342.
17. Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014; 3:458–468. PMID: 26280007.

18. Sadowski EA, Bennett LK, Chan MR, Wentland AL, Garrett AL, Garrett RW, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007; 243:148–157. PMID: 17267695.

19. Pocha C, Dieperink E, McMaken KA, Knott A, Thuras P, Ho SB. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography -- a randomised study. Aliment Pharmacol Ther. 2013; 38:303–312. PMID: 23750991.

20. Lim KS. Diffusion-weighted MRI of hepatocellular carcinoma in cirrhosis. Clin Radiol. 2014; 69:1–10. PMID: 24034549.

21. Nasu K, Kuroki Y, Nawano S, Kuroki S, Tsukamoto T, Yamamoto S, et al. Hepatic metastases: diffusion-weighted sensitivity-encoding versus SPIO-enhanced MR imaging. Radiology. 2006; 239:122–130. PMID: 16493012.

22. Park MJ, Kim YK, Lee MW, Lee WJ, Kim YS, Kim SH, et al. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology. 2012; 264:761–770. PMID: 22843769.

23. Yoo H, Lee JM, Yoon JH, Kang HJ, Lee SM, Yang HK, et al. T2* mapping from multi-echo dixon sequence on gadoxetic acid-enhanced magnetic resonance imaging for the hepatic fat quantification: can it be used for hepatic function assessment? Korean J Radiol. 2017; 18:682–690. PMID: 28670163.

24. Xie S, Li Q, Cheng Y, Zhang Y, Zhuo Z, Zhao G, et al. Impact of liver fibrosis and fatty liver on T1rho measurements: a prospective study. Korean J Radiol. 2017; 18:898–905. PMID: 29089822.

25. Ahn JH, Yu JS, Cho ES, Chung JJ, Kim JH, Kim KW. Diffusion-weighted MRI of malignant versus benign portal vein thrombosis. Korean J Radiol. 2016; 17:533–540. PMID: 27390544.

26. Lee GM, Kim YR, Ryu JH, Kim TH, Cho EY, Lee YH, et al. Quantitative measurement of hepatic fibrosis with gadoxetic acid-enhanced magnetic resonance imaging in patients with chronic hepatitis B infection: a comparative study on aspartate aminotransferase to platelet ratio index and fibrosis-4 index. Korean J Radiol. 2017; 18:444–451. PMID: 28458596.

27. Yoon JH, Lee JM, Yu MH, Kim EJ, Han JK. Triple arterial phase MR imaging with gadoxetic acid using a combination of contrast enhanced time robust angiography, keyhole, and viewsharing techniques and two-dimensional parallel imaging in comparison with conventional single arterial phase. Korean J Radiol. 2016; 17:522–532. PMID: 27390543.

28. Hardie AD, Kizziah MK, Rissing MS. Can the patient with cirrhosis be imaged for hepatocellular carcinoma without gadolinium?: comparison of combined T2-weighted, T2*-weighted, and diffusion-weighted MRI with gadolinium-enhanced MRI using liver explantation standard. J Comput Assist Tomogr. 2011; 35:711–715. PMID: 22082541.
29. Lin OS, Keeffe EB, Sanders GD, Owens DK. Cost-effectiveness of screening for hepatocellular carcinoma in patients with cirrhosis due to chronic hepatitis C. Aliment Pharmacol Ther. 2004; 19:1159–1172. PMID: 15153169.

30. Arguedas MR, Chen VK, Eloubeidi MA, Fallon MB. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: a cost-utility analysis. Am J Gastroenterol. 2003; 98:679–690. PMID: 12650806.

31. Andersson KL, Salomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2008; 6:1418–1424. PMID: 18848905.

32. Cucchetti A, Trevisani F, Cescon M, Ercolani G, Farinati F, Poggio PD, et al. Italian Liver Cancer (ITA.LI.CA) Group. Cost-effectiveness of semi-annual surveillance for hepatocellular carcinoma in cirrhotic patients of the Italian Liver Cancer population. J Hepatol. 2012; 56:1089–1096. PMID: 22245900.

33. Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med. 1996; 101:422–434. PMID: 8873514.

34. Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001; 48:251–259. PMID: 11156649.

Fig. 2
61-year-old man with HCC in S8.
Case with typical findings favoring HCC on non-contrast MRI.
On T2-weighted Half-Fourier Acquisition Single-shot Turbo spin Echo image, there is subtle high signal mass in S8 of liver (arrow, A), with diffusion restriction (arrow, B) on DWI (b = 500 s/mm2). After gadoxetic acid injection, this mass displays prominent arterial enhancement (arrow, C). On portal venous phase, this mass exhibits washout and ring-like enhancement (arrow, D). These are typical non-enhanced and enhanced magnetic resonance findings of HCC. DWI = diffusion weighted imaging
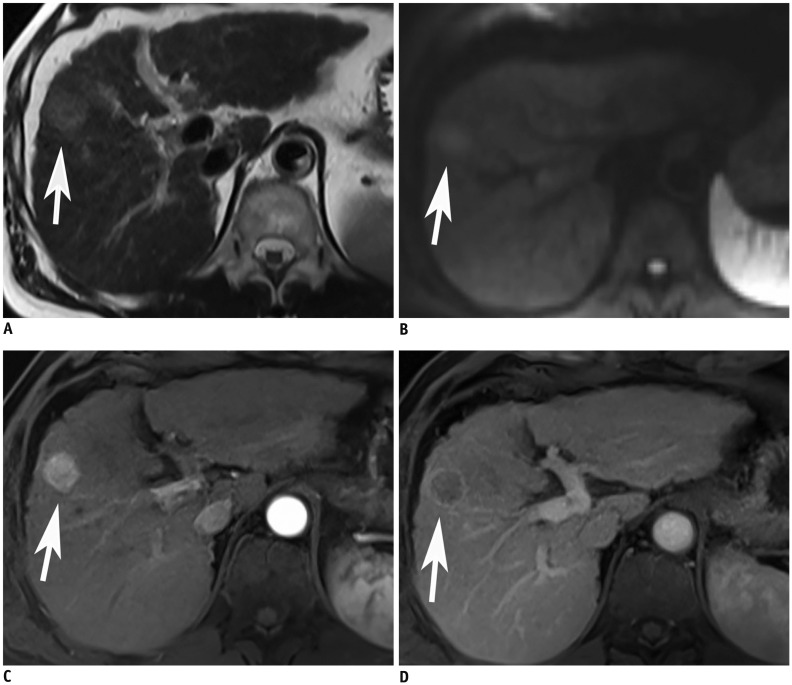
Fig. 3
81-year-old man with HCC in S7, which was not detect on non-contrast MRI.
On arterial phase image after gadoxetic acid injection, there is well-enhancing mass (arrow, A) with washout on portal venous phase (arrow, B) in S7 that can be diagnosed as HCC. However, this mass cannot be detected on T2 weighted fast spin echo image due to iso-intensity signal to that of liver parenchyma (arrow, C). Also, diffusion restriction is not clear (b = 500 s/mm2) (arrow, D). T1WI displays subtle high signal intensity of this mass (arrow, E). Both reviewers considered this mass as dysplastic nodule because of iso-intensity signal on T2WI and high signal on T1WI. T1WI = T1-weighted image, T2WI = T2-weighted image
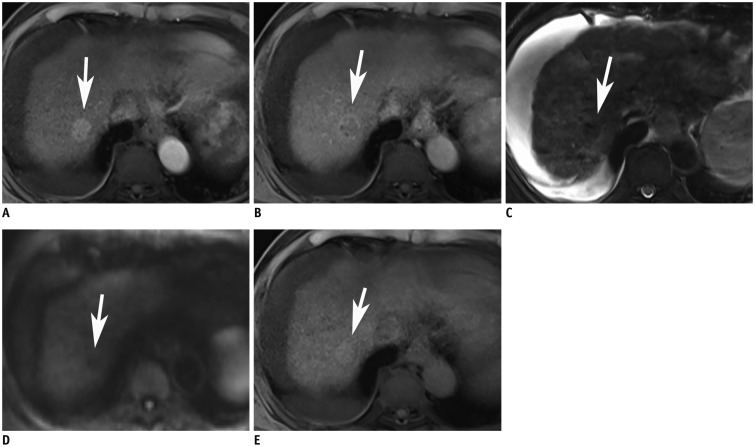
Fig. 4
65-year-old man with hemangioma.
False positive case.
There is small nodular lesion with high signal intensity on T2WI in left lateral sector of liver (arrow, A). On DWI (b = 500 s/mm2), this lesion shows diffusion restriction (arrow, B). However, this lesion shows nodular enhancement on arterial phase (arrow, C) and entire enhancement of lesion (arrow, D). This case of hemangioma was misdiagnosed as HCC by both readers.
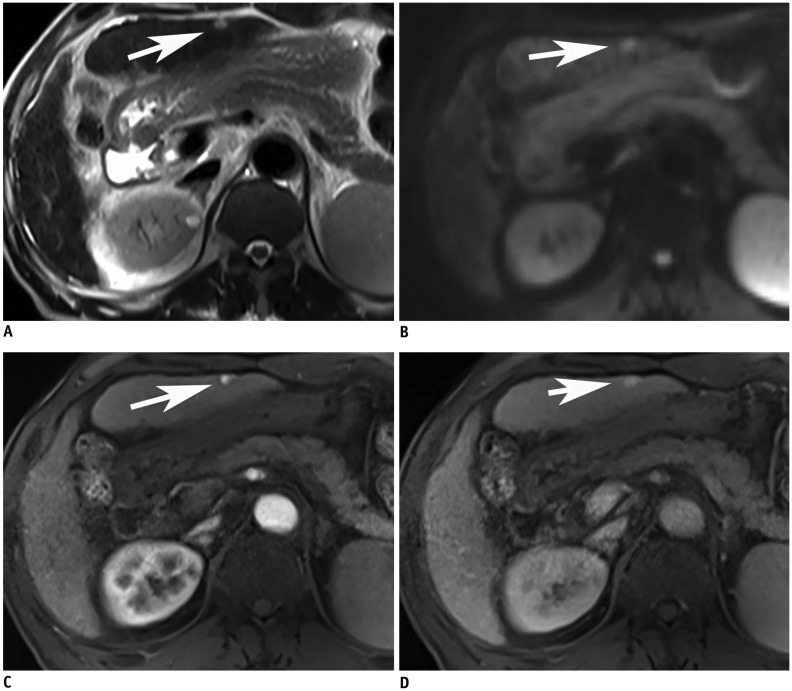
Table 1
Characteristics of Study Population
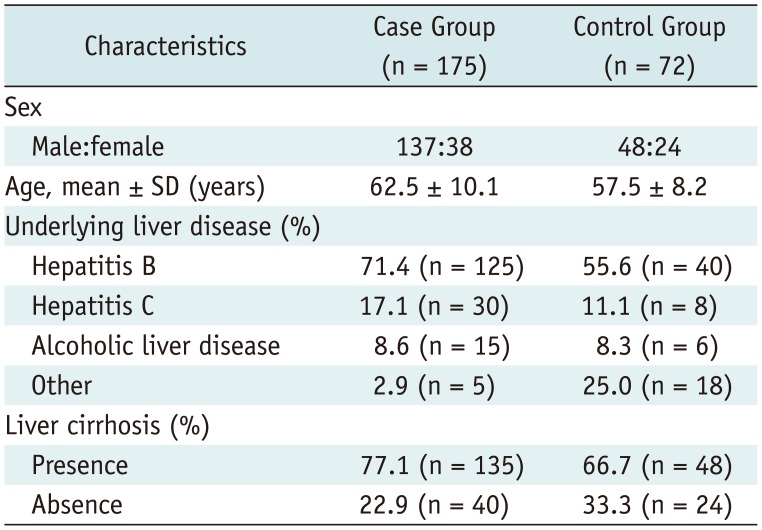
Table 2
MRI Pulse Sequences
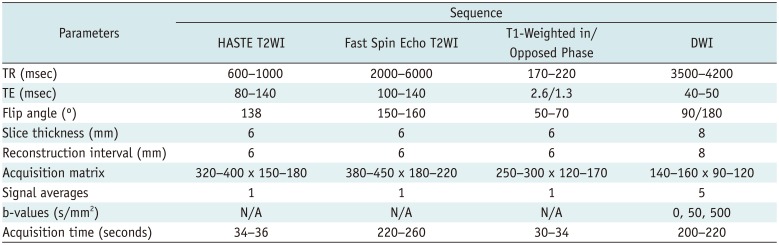
Table 3
Per-Patient Diagnostic Performance of Non-Contrast MRI




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download