Abstract
Objective
None of the previous studies have investigated the interval change in ultrasonography (US) features of solid thyroid nodules (STNs) after US-guided fine-needle aspiration (US-FNA). This study aimed to assess the prevalence and characteristics of US interval changes in STNs after US-FNA.
Materials and Methods
This study included 257 STNs in 257 patients in whom thyroid US and initial US-FNA had been performed by two radiologists from January 2015 to June 2015. One of the radiologists performed single needle puncture in all cases, whereas the other radiologist used double or triple needle punctures. Follow-up US examinations were performed after 12.0 ± 6.0 months. We evaluated the prevalence and characteristics of post-FNA US interval changes through a retrospective analysis. In addition, multiple factors were correlated with post-FNA US interval changes.
Results
The number of needle punctures was one (n = 91), two (n = 163), and three (n = 3). Of the 257 STNs (mean diameter, 11.9 mm) in 257 patients, 35 (13.6%) showed an interval change in US features on follow-up US. Among them, 17 STNs (6.6%) showed newly developed malignant US features, including hypoechogenicity (n = 5), microcalcifications (n = 2), a spiculated margin (n = 4), hypoechogenicity with a spiculated margin (n = 5), and microcalcifications with non-parallel orientation (n = 1). Between patients who showed presence and absence of US interval changes, there were no significant differences in patient age, sex, nodule size, dichotomization, and location, Korean Thyroid Imaging Reporting and Data System categorization after FNA, practitioners involved, number of needle punctures, cytological findings, and interval between FNA and US follow-up (p > 0.05).
Ultrasonography (US) is the primary imaging modality for evaluating nodular thyroid lesions (1). Thyroid nodules are very commonly identified by US in 10−41% of the individuals, and the prevalence of thyroid nodules increases with age (1). In the literature, malignant US features of thyroid nodules include marked hypoechogenicity, a spiculated margin, microcalcifications, and a taller-than-wide shape (12). Recently, the Korean Society of Thyroid Radiology suggested a new risk stratification system for thyroid nodules, the Korean Thyroid Imaging Reporting and Data System (K-TIRADS), which includes three suspicious US features (microcalcifications, non-parallel orientation, and a spiculated/microlobulated margin) and risk stratification according to the combination of nodule solidity and echogenicity (3). In practice, when a thyroid nodule with malignant US features is detected by thyroid US, US-guided fine-needle aspiration (US-FNA) is first used for further evaluation (24). However, the diagnostic accuracy of US-FNA for thyroid nodules varies, ranging from 60% to 96% (456). Furthermore, it is operator-dependent, shows indeterminate cytology, and has associated complications (1456). In addition, needle aspiration can result in varying degrees of tissue injury (7).
According to the literature, the trauma induced by the aspiration needle can lead to tissue changes of varying severity, and it occasionally causes problems in histological assessment of thyroid nodules (789). These histological changes include hemorrhage, infarction, granulation tissue formation, vascular change, spindle cell proliferation, and capsular and vascular pseudoinvasion (89). Among them, the three most common post-FNA changes that lead to requests for pathology consultation are infarction, vascular changes, and nuclear atypia (7).
To date, few imaging studies of post-FNA changes in benign thyroid nodules have been reported (10111213). The procedure-related scar tissue of thyroid nodules can show marked hypoechogenicity with irregular borders on US (14). In benign thyroid nodules with post-FNA changes, only meticulous comparison with previous images showing nodule shrinkage over time is useful for achieving the correct diagnosis (1011121314). Unfortunately, unnecessary biopsy or surgery for these nodules is performed in practice. In a previous study, core needle biopsy was performed for thyroid nodules with malignant US features and size reduction, and upon histological examination, these nodules were primarily composed of internal fibrosis and hemorrhage (15).
However, to the best of our knowledge, none of the studies have compared the prevalence and characteristics of post-FNA US interval changes according to needle puncture and other factors in solid thyroid nodules (STNs, defined as thyroid nodules with a solid component accounting for ≥ 90% of the total volume). The aim of this study was to evaluate the prevalence and characteristics of US interval changes in STNs after US-FNA. In addition, we attempted to assess the differences in the prevalence and characteristics of US interval changes in STNs, according to the number of needle punctures used and the practitioners involved.
This retrospective study was approved by the relevant Institutional Review Board, and the requirement for obtaining informed consent was waived (IRB No. 16-0231). From January 2015 to June 2015, two radiologists (who had performed > 300 US-FNAs/year for thyroid nodules over a period of 13 years and 5 years, respectively) performed thyroid US and an initial US-FNA to diagnose 414 STNs (mean size, 11.9 ± 9.1 mm; range, 2.9−67.9 mm) in 414 patients (355 female subjects and 59 male subjects; mean age, 52.3 ± 11.1 years; range, 17−81 years). Because of thyroid surgery (n = 72), loss to follow-up (n = 80), and poor US image quality (n = 5), 157 STNs (mean diameter, 11.9 ± 9.7 mm; range, 2.9−49.6 mm) were excluded from the study. Ultimately, 257 STNs (mean diameter, 11.8 ± 8.8 mm; range, 3.0−67.0 mm) in 257 patients (230 female subjects and 27 male subjects; mean age, 52.8 ± 10.6 years; range, 25−81 years) were included in this study. Of the 257 STNs, 119 were subcentimeter in size. The reasons for performing US-FNA for subcentimeter nodules included suspicious US features (n = 56), presence of contralateral thyroid malignancy (n = 18), and patients' requests (n = 45).
Thyroid US was performed by two radiologists using a high-resolution ultrasound instrument (iU 22; Philips Medical Systems, Bothell, WA, USA) equipped with a 12−15 MHz linear probe. US-FNA was performed immediately after thyroid US by the same radiologist, using the same sampling technique, a 23-gauge needle, without an aspiration device, and without local anesthesia (16). The more experienced radiologist performed only single needle puncture in all cases, whereas the other radiologist used double or triple needle punctures. For each sample, a smear was prepared on four to six slides, using the flipping-extraction technique; it was fixed in 95% ethanol and examined after Papanicolaou staining.
Cytological findings were categorized as follows: inadequate (Bethesda class I), benign (Bethesda class II), atypia of undetermined significance or follicular lesion of undetermined significance (Bethesda class III), suspicious for a follicular neoplasm (Bethesda class IV), suspicious for malignancy (Bethesda class V), or malignant (Bethesda class VI) (17).
In December 2016, a single radiologist retrospectively investigated all US features of STNs using a picture archiving and communication system. The mean interval between US-FNA and the first follow-up US was 12.0 ± 6.0 months (range: 1–24 months). The imaging features of STNs on initial US before US-FNA and on follow-up US were compared in each case. On image analysis, echogenicity, margin, shape, and vascularity of STNs were investigated. Echogenicity was classified as iso- (the same echogenicity as that of the adjacent thyroid parenchyma), hypo- (decreased echogenicity as compared to that of the adjacent thyroid parenchyma), and hyper- (increased echogenicity as compared to that of the adjacent thyroid parenchyma). STN margin was classified as smooth, spiculated, lobulated, or poorly defined. STN shape was classified as ovoid-to-round or irregular. The orientation of a STN was classified as parallel (when the anteroposterior diameter of the STN was equal to or less than its transverse or longitudinal diameter) or non-parallel (when the anteroposterior diameter of the STN was longer than its transverse or longitudinal diameter in a transverse or longitudinal plane).
On the basis of a retrospective analysis of US features, each STN was classified into K-TIRADS category 3 to 5 (2). Suspicious features included microcalcification, a spiculated/microlobulated margin, and non-parallel orientation. STNs with isoechogenicity or hyperechogenicity and no suspicious US features were categorized as K-TIRADS category 3 (low suspicion). STNs with hypoechogenicity and no suspicious US features were categorized as K-TIRADS category 4 (intermediate suspicion). STNs with any of the suspicious US features were categorized as K-TIRADS category 5 (high suspicion).
The normal distribution of continuous data was tested with the Kolmogorov-Smirnov test. Normally distributed variables, including age, size of thyroid nodules, and interval of follow-up US were compared by using independent t tests, and they were then expressed as the mean ± SD. Group comparisons of categorical variables were performed using the chi-square test, or Fisher's exact test for small cell values. The linear-by-linear association (or χ2 test for trend) was used to evaluate the linear association between the post-FNA US interval change of STNs and the initial K-TIRADS category of STNs. All statistical analyses were performed using the IBM SPSS Statistics 19.0 software package (IBM Corp., Armonk, NY, USA), with p < 0.05 indicating statistical significance.
For the 257 STNs in 257 patients, one or more sessions of follow-up US were performed after US-FNA (Fig. 1). The mean diameter of STNs < 10 mm (n = 119) and ≥ 10 mm (n = 138) in the largest diameter was 5.9 ± 1.8 mm and 17.1 ± 9.1 mm, respectively. The location of 257 STNs included the right lobe (n = 119), the left lobe (n = 129), and the isthmus (n = 9). The number of needle punctures used were one (n = 91), two (n = 163), and three (n = 3). US categories of the 257 STNs included benign (n = 77), indeterminate (n = 141), and malignant (n = 39). The Bethesda classes of these 257 STNs were I (n = 6), II (n = 199), III (n = 39), IV (n = 2), V (n = 4), and VI (n = 7).
Of the 257 STNs, 35 (13.6%) showed an interval change in US features on follow-up US (Figs. 2, 3). Of the 35 STNs with post-FNA US interval change, 17 (6.6%) showed new malignant US features on follow-up US; hypoechogenicity (n = 5), microcalcifications (n = 2), a spiculated margin (n = 4), hypoechogenicity with a spiculated margin (n = 5), and microcalcifications with non-parallel orientation (n = 1). In the 18 STNs (7.0%) without any malignant US features on follow-up US, new detection of intranodular cystic component (n = 9), intranodular hypoechoic component (n = 8), and loss of known hypoechogenicity (n = 1) were noted.
Before US-FNA, K-TIRADS categories 3 (n = 69), 4 (n = 102), and 5 (n = 86) were noted (Table 1). On follow-up US after US-FNA, K-TIRADS categories 3 (n = 68), 4 (n = 96), and 5 (n = 93) were noted. On follow-up US after US-FNA, 12 STNs (4.7%) showed interval changes in K-TIRADS category, whereas 245 STNs showed no interval change. However, there was no significant linear association between the post-FNA US interval changes and the initial K-TIRADS of STNs (p = 0.071). Of the 12 STNs with an interval change in the K-TIRADS category, 11 showed a higher category on follow-up US, whereas 1 STN revealed a lower category. When the interval change in the K-TIRADS category was compared in accordance with the number of needle punctures, there was no significant difference (p = 0.681).
The comparison of clinico-pathological data of STNs according to US interval changes is shown in Table 2. Between STNs with and without US interval changes after US-FNA, there was a significant difference in K-TIRADS categorization before US-FNA (p = 0.022). In the majority of cases, post-FNA US interval change was observed in category 4. However, there were no significant differences in the patients' age or sex, size, dichotomization (< 10 mm and ≥ 10 mm), or location of the nodule, the practitioners involved, number of needle punctures involved, cytological findings, interval between the FNA and US follow-up, and K-TIRADS categorization after US-FNA (p > 0.05).
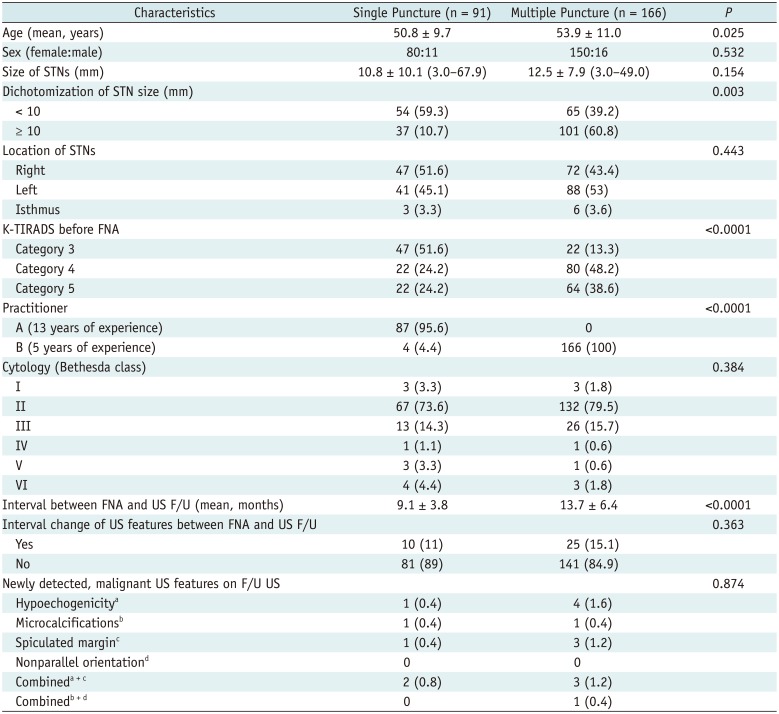
The comparison of clinico-pathological and US data of STNs according to the number of needle punctures used is presented in Table 3. Between the single and multiple needle puncture groups, there were statistically significant differences in patient age (p = 0.025), nodule dichotomization (p = 0.003), K-TIRADS categorization before US-FNA (p < 0.0001), practitioners involved (p < 0.0001), the interval between US-FNA and US follow-up (p < 0.0001), whereas there were no significant differences in terms of sex (p = 0.532), size (p = 0.154) or location (p = 0.443) of the nodules, cytological findings (p = 0.384), interval changes in US features (p = 0.363), and the presence of newly detected malignant US features (p = 0.874).
For the 257 STNs, one practitioner used a single needle puncture in all cases (n = 91), whereas the other practitioner used double (n = 163) or triple (n = 3) needle punctures. Between the two practitioners, there were statistically significant differences in terms of patient age (p = 0.009), number of needle punctures used (p < 0.0001), and interval between US-FNA and US follow-up (p < 0.0001). In contrast, no significant differences were found in terms of sex (p = 0.424), size (p = 0.326) or location (p = 0.398) of the nodules, cytological findings (p = 0.298), interval changes in US features (p = 0.274), and the presence of newly detected malignant US features (p = 0.822).
Ultrasonography-guided fine-needle aspiration is the first-line diagnostic method for evaluating thyroid nodules. However, variable changes can occur in thyroid nodules after FNA, which may influence cytological analyses (717). Post-FNA changes in thyroid nodules are classified as acute and chronic types; acute changes include hemorrhage, granulation tissue formation, and nuclear atypia, whereas chronic changes include capsular distortion, vascular pseudoinvasion, and papillary endothelial hyperplasia (7). Post-FNA changes rarely replace a thyroid nodule completely or extensively (18). In such a case, US features of a thyroid nodule can be altered. In practice, when a benign thyroid nodule shows malignant US features on follow-up US, because of post-FNA changes, unnecessary repeated FNA or core needle biopsy cannot be avoided. In the present study, 13.6% (35/257) of STNs showed US interval changes after US-FNA. Among them, 48.6% (17/35) revealed newly developed malignant US features, such as hypoechogenicity, microcalcification, a spiculated/microlobulated margin, non-parallel orientation, and combination of these malignant findings. However, only 12 STNs (4.7%) showed an interval change in the K-TIRADS category on follow-up US, although most of them changed to a higher K-TIRADS category.
In the 17 STNs with newly developed malignant US features, the prevalence rate of hypoechogenicity and a spiculated margin was 58.8% (10/17) and 52.9% (9/17), respectively, whereas the prevalence rate of microcalcifications and non-parallel orientation was 17.6% (3/17) and 5.9% (1/17), respectively. This may be the characteristics of post-FNA changes rather than thyroid malignancy. However, the present study showed a low prevalence of STN cases with newly developed malignant US features. Further studies including larger sample sizes are required to clarify this discrepancy. Nevertheless, awareness of US interval changes in STNs before and after US-FNA is essential for differentiating these changes from thyroid malignancies. In particular, the interval decrease in the nodule size before and after US-FNA of thyroid nodules suggests benignity despite the presence of malignant US features (1011121315).
The size of the FNA needle and the number of needle punctures used may influence the post-FNA changes or US interval changes in STNs. Previous studies have reported that needle injury is known to be closely associated with post-FNA changes (19). In terms of the pathogenic mechanism, needle injury causes direct interruption of the microvascular supply and venous thrombosis (789). However, in the study, there was no significant difference in US interval changes between the single and multiple needle puncture groups. The reason for this occurrence is unclear, but it may be because the majority of post-FNA changes were resolved before US follow-up and because a same size FNA needle was used in our study. Other than the needle factor, the aspiration technique (capillary and aspiration), the number of to-and-fro motions, the level of experience of the practitioner, or nodule biology may influence post-FNA changes or US interval changes. In particular, the experience level of the practitioner may be an important factor in post-FNA changes. However, in the present study, there was no significant difference in US interval changes in STNs between the two practitioners who had different levels of experience with US-FNA.
In addition to the intrinsic limitations of a retrospective study, there were several limitations in the present study. First, of the 414 STNs, 157 (37.9%) were excluded from the study because of no US follow-up or poor US image quality. Second, there was a lack of systematic strategies for follow-up US owing to the variable frequency and interval of follow-up US. For clarity, a further study including regular follow-up US at short intervals should be performed. Third, a retrospective analysis of all US images of follow-up US was performed by a single radiologist. Furthermore, nodule vascularity was not evaluated. Finally, the presence of underlying diffuse thyroid disease was not evaluated.
In conclusion, this study demonstrated that the prevalence of US interval changes in STNs after US-FNA was 13.6% (35/257) and that approximately one-third (12/35) of the STNs with US interval changes showed newly developed malignant US features. Therefore, awareness of US interval changes in STNs after US-FNA may be helpful for managing STNs and it can avoid unnecessary biopsies.
References
1. Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005; 237:794–800. PMID: 16304103.

2. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: Revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016; 17:370–395. PMID: 27134526.

3. Ha EJ, Moon WJ, Na DG, Lee YH, Choi N, Kim SJ, et al. A Multicenter prospective validation study for the Korean thyroid imaging reporting and data system in patients with thyroid nodules. Korean J Radiol. 2016; 17:811–821. PMID: 27587972.

4. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133. PMID: 26462967.

5. Lee YH, Baek JH, Jung SL, Kwak JY, Kim JH, Shin JH, et al. Ultrasound-guided fine needle aspiration of thyroid nodules: a consensus statement by the Korean Society of Thyroid Radiology. Korean J Radiol. 2015; 16:391–401. PMID: 25741201.

6. Kim DW, Lee EJ, Kim SH, Kim TH, Lee SH, Kim DH, et al. Ultrasound-guided fine-needle aspiration biopsy of thyroid nodules: comparison in efficacy according to nodule size. Thyroid. 2009; 19:27–31. PMID: 19021460.

7. Baloch ZW, LiVolsi VA. Post fine-needle aspiration histologic alterations of thyroid revisited. Am J Clin Pathol. 1999; 112:311–316. PMID: 10478135.

8. LiVolsi VA, Merino MJ. Worrisome histologic alterations following fine-needle aspiration of the thyroid (WHAFFT). Pathol Annu. 1994; 29:99–120. PMID: 7936753.
9. Kini SR. Post-fine-needle biopsy infarction of thyroid neoplasms: a review of 28 cases. Diagn Cytopathol. 1996; 15:211–220. PMID: 8955603.

10. Ko MS, Jeong KS, Shong YK, Gong GY, Baek JH, Lee JH. Collapsing benign cystic nodules of the thyroid gland: sonographic differentiation from papillary thyroid carcinoma. AJNR Am J Neuroradiol. 2012; 33:124–127. PMID: 22158923.

11. Kim DW. Benign lesions that mimic thyroid malignancy on ultrasound. Can Assoc Radiol J. 2015; 66:79–85. PMID: 24931046.

12. Lacout A, Chevenet C, Marcy PY. Mummified thyroid syndrome. AJR Am J Roentgenol. 2016; 206:837–845. PMID: 27003052.

13. Sohn YM, Kim EK, Moon HJ, Kim SJ, Kwak JY. Suspiciously malignant findings on ultrasound after fine needle aspiration biopsy in a thyroid nodule with initially benign ultrasound and cytologic result: to repeat or to follow-up. Clin Imaging. 2011; 35:470–475. PMID: 22040793.

14. Russ G. Risk stratification of thyroid nodules on ultrasonography with the French TI-RADS: description and reflections. Ultrasonography. 2016; 35:25–38. PMID: 26324117.

15. Lee HY, Baek JH, Ha EJ, Park JW, Lee JH, Song DE, et al. Malignant-looking thyroid nodules with size reduction: core needle biopsy results. Ultrasonography. 2016; 35:327–334. PMID: 27184652.

16. Kim DW, Choo HJ, Park JS, Lee EJ, Kim SH, Jung SJ, et al. Ultrasonography-guided fine-needle aspiration cytology for thyroid nodules: an emphasis on one-sampling and biopsy techniques. Diagn Cytopathol. 2012; 40(Suppl 1):E48–E54. PMID: 21416648.

17. Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid. 2009; 19:1159–1165. PMID: 19888858.

18. Eze OP, Cai G, Baloch ZW, Khan A, Virk R, Hammers LW, et al. Vanishing thyroid tumors: a diagnostic dilemma after ultrasonography-guided fine-needle aspiration. Thyroid. 2013; 23:194–200. PMID: 22928739.

19. Kholová I. Vanishing thyroid gland tumors: Infarction as consequence of FNA. Diagn Cytopathol. 2016; 44:568–573. PMID: 27094979.

Fig. 1
Diagram illustrating enrollment of subjects and data analysis using study's algorithm.
STNs = solid thyroid nodules, US = ultrasonography, US-FNA = US-guided fine-needle aspiration
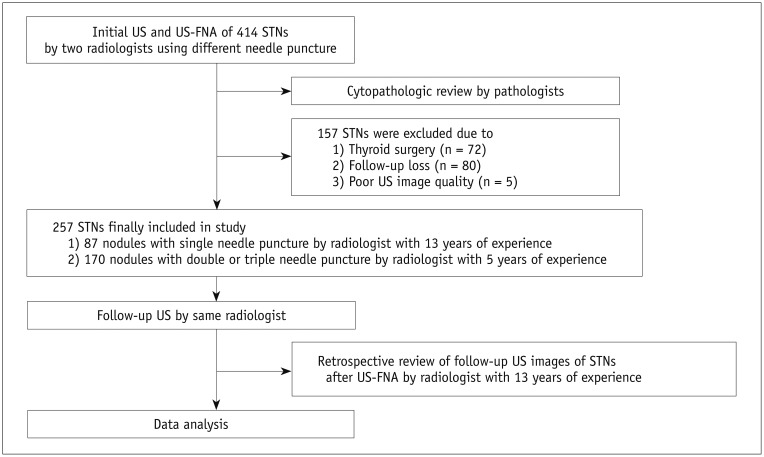
Fig. 2
55-year-old woman showing interval changes in US features of STN after US-FNA performed using double needle puncture.
Before US-FNA, transverse (A) and longitudinal (B) gray-scale sonograms show STN with round shape in right thyroid lobe (arrows, 4.5 × 4.7 × 5.4 mm; K-TIRADS category 3). Radiologist with 5 years of experience performed US-FNA of this nodule, using double needle puncture. Cytological examination revealed that nodule belonged to Bethesda class II. At 12-month follow-up US after US-FNA, transverse (C) and longitudinal (D) gray-scale sonograms showed US interval changes in nodule (arrows). This nodule showed hypoechogenicity and spiculated margin, whereas nodule size decreased (3.7 × 3.8 × 4.6 mm; K-TIRADS category 5). Cytological examination after repeated US-FNA of this nodule revealed Bethesda class II. K-TIRADS = Korean Thyroid Imaging Reporting and Data System
Fig. 3
56-year-old woman with interval changes in US features of STN after US-FNA performed using single needle puncture.
Before US-FNA, transverse (A) and longitudinal (B) gray-scale sonograms show STN with benign US features in left thyroid lobe (arrows, 6.0 × 10.4 × 11.2 mm; K-TIRADS category 3). Radiologist with 13 years of experience performed US-FNA of this nodule using single needle puncture. Cytological examination revealed that nodule belonged to Bethesda class II. At 12-month follow-up US after US-FNA, transverse (C) and longitudinal (D) gray-scale sonograms show US interval changes in nodule (arrows). This nodule showed decreased echogenicity and lobulated margin, whereas nodule size decreased (5.9 × 8.4 × 9.6 mm; K-TIRADS category 5). On same day, repeated US-FNA was performed for this nodule. Cytological examination revealed that nodule belonged to Bethesda class II.

Table 1
Interval Change of K-TIRADS Categorization after FNA of Solid Thyroid Nodules according to Number of Needle Puncture
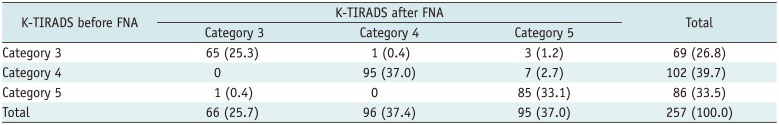
Table 2
Comparison of Clinico-Pathologic Data of 257 STNs according to Interval Changes in US Features
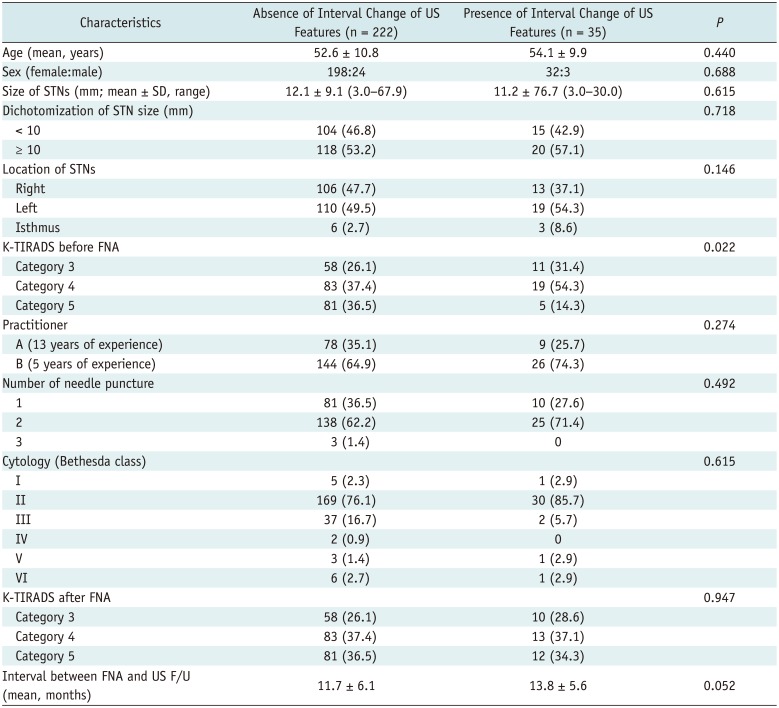
Table 3
Comparison of Clinico-Pathologic and US Data of 257 STNs according to Number of Needle Punctures Used




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download