1. Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992; 16:1343–1349.
2. Saad WE, Al-Osaimi AM, Caldwell S, Ray CE, Lorenz JM, Burke CT, et al. ACR appropriateness criteria: radiologic management of gastric varices. Expert Panel on Interventional Radiology, American College of Radiology 2012. Web site. Accessed January 24, 2017.
https://acsearch.acr.org/docs/70911/Narrative/.
3. Saad WE, Simon PO Jr, Rose SC. Balloon-occluded retrograde transvenous obliteration of gastric varices. Cardiovasc Intervent Radiol. 2014; 37:299–315.
4. Kerlan RK Jr, LaBerge JM, Baker EL, Wack JP, Marx M, Somberg KA, et al. Successful reversal of hepatic encephalopathy with intentional occlusion of transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 1995; 6:917–921.
5. Brown RS Jr, Lake JR. Transjugular intrahepatic portosystemic shunt as a form of treatment for portal hypertension: indications and contraindications. Adv Intern Med. 1997; 42:485–504.
6. Sanyal AJ, Freedman AM, Luketic VA, Purdum PP 3rd, Shiffman ML, DeMeo J, et al. The natural history of portal hypertension after transjugular intrahepatic portosystemic shunts. Gastroenterology. 1997; 112:889–898.
7. Katoh K, Sone M, Hirose A, Inoue Y, Fujino Y, Onodera M. Balloon-occluded retrograde transvenous obliteration for gastric varices: the relationship between the clinical outcome and gastrorenal shunt occlusion. BMC Med Imaging. 2010; 10:2.
8. Elsamman MK, Fujiwara Y, Kameda N, Okazaki H, Tanigawa T, Shiba M, et al. Predictive factors of worsening of esophageal varices after balloon-occluded retrograde transvenous obliteration in patients with gastric varices. Am J Gastroenterol. 2009; 104:2214–2221.
9. Choi YH, Yoon CJ, Park JH, Chung JW, Kwon JW, Choi GM. Balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding: its feasibility compared with transjugular intrahepatic portosystemic shunt. Korean J Radiol. 2003; 4:109–116.
10. Ninoi T, Nakamura K, Kaminou T, Nishida N, Sakai Y, Kitayama T, et al. TIPS versus transcatheter sclerotherapy for gastric varices. AJR Am J Roentgenol. 2004; 183:369–376.
11. Sabri SS, Abi-Jaoudeh N, Swee W, Saad WE, Turba UC, Caldwell SH, et al. Short-term rebleeding rates for isolated gastric varices managed by transjugular intrahepatic portosystemic shunt versus balloon-occluded retrograde transvenous obliteration. J Vasc Interv Radiol. 2014; 25:355–361.
12. Chau TN, Patch D, Chan YW, Nagral A, Dick R, Burroughs AK. “Salvage” transjugular intrahepatic portosystemic shunts: gastric fundal compared with esophageal variceal bleeding. Gastroenterology. 1998; 114:981–987.
13. Barange K, Péron JM, Imani K, Otal P, Payen JL, Rousseau H, et al. Transjugular intrahepatic portosystemic shunt in the treatment of refractory bleeding from ruptured gastric varices. Hepatology. 1999; 30:1139–1143.
14. Rees CJ, Nylander DL, Thompson NP, Rose JD, Record CO, Hudson M. Do gastric and oesophageal varices bleed at different portal pressures and is TIPS an effective treatment? Liver. 2000; 20:253–256.
15. Sarin SK, Kumar A. Gastric varices: profile, classification, and management. Am J Gastroenterol. 1989; 84:1244–1249.
16. Haskal ZJ, Rees CR, Ring EJ, Saxon R, Sacks D. Reporting standards for transjugular intrahepatic portosystemic shunts. Technology Assessment Committee of the SCVIR. J Vasc Interv Radiol. 1997; 8:289–297.
17. Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002; 35:716–721.
18. Saad WE, Kitanosono T, Koizumi J, Hirota S. The conventional balloon-occluded retrograde transvenous obliteration procedure: indications, contraindications, and technical applications. Tech Vasc Interv Radiol. 2013; 16:101–151.
19. Patel A, Fischman AM, Saad WE. Balloon-occluded retrograde transvenous obliteration of gastric varices. AJR Am J Roentgenol. 2012; 199:721–729.
20. Borghei P, Kim SK, Zuckerman DA. Balloon occlusion retrograde transvenous obliteration of gastric varices in two non-cirrhotic patients with portal vein thrombosis. Korean J Radiol. 2014; 15:108–113.
21. Bureau C, Garcia-Pagan JC, Otal P, Pomier-Layrargues G, Chabbert V, Cortez C, et al. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized study. Gastroenterology. 2004; 126:469–475.
22. Saad WE, Darcy MD. Transjugular intrahepatic portosystemic shunt (TIPS) versus balloon-occluded retrograde transvenous obliteration (BRTO) for the management of gastric varices. Semin Intervent Radiol. 2011; 28:339–349.
23. Jang SY, Kim GH, Park SY, Cho CM, Tak WY, Kim JH, et al. Clinical outcomes of balloon-occluded retrograde transvenous obliteration for the treatment of gastric variceal hemorrhage in Korean patients with liver cirrhosis: a retrospective multicenter study. Clin Mol Hepatol. 2012; 18:368–374.
24. Watanabe M, Shiozawa K, Ikehara T, Nakano S, Kougame M, Otsuka T, et al. Short-term effects and early complications of balloon-occluded retrograde transvenous obliteration for gastric varices. ISRN Gastroenterol. 2012; 2012:919371.
25. Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol. 2001; 12:327–336.
26. Chao Y, Lin HC, Lee FY, Wang SS, Tsai YT, Hsia HC, et al. Hepatic hemodynamic features in patients with esophageal or gastric varices. J Hepatol. 1993; 19:85–89.
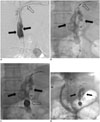
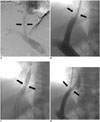




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download