Abstract
T-cell non-Hodgkin lymphomas (NHLs) are biologically diverse, uncommon malignancies characterized by a spectrum of imaging findings according to subtype. The purpose of this review is to describe the common subtypes of T-cell NHL, highlight important differences between cutaneous, various peripheral and precursor subtypes, and summarize imaging features and the role of imaging in the management of this diverse set of diseases.
T-cell non-Hodgkin lymphomas (NHLs) are biologically diverse, uncommon malignancies that represent approximately 12% of all NHLs (1). T-cell NHL includes a spectrum of disease most recently defined in 2016, according to the World Health Organization (WHO)/European Organization for Research and Treatment of Cancer classification (2). In the United States, cutaneous T-cell lymphoma (CTCL) and peripheral T-cell lymphoma (PTCL), not otherwise specified (NOS) are the most common mature T-cell NHL subtypes, accounting for 26.5% and 15% of cases, respectively (345). Mature T-cell NHLs may be indolent or aggressive; CTCLs are generally indolent, while PTCL-NOS is a “wastebasket” category of aggressive nodal lymphoma. Precursor T-cell lymphoblastic lymphoma (T-LBL) is a highly aggressive disease, representing 14% of lymphoblastic lymphoma typically characterized separately from mature T-cell NHL subtypes (5). Numerous other T-cell NHL subtypes have been described, each with characteristic histopathologic features and clinical behavior.
Imaging plays an important role in staging and management of many T-cell NHLs. CT is the most commonly used tool for staging and follow-up as it is readily available, easy to perform, reliable and reproducible (6). However, nodal involvement by CT is limited to assessment of node size and shape, and it may poorly depict involvement of bone marrow and some extranodal sites (78). 18F-flourodeoxyglucose (FDG) positron emission tomography CT (PET-CT) is increasingly used for staging and response assessment, particularly for aggressive lymphomas due to its superior sensitivity. PET-CT improves detection of extranodal involvement and accuracy of baseline staging compared with CT alone (910). Evidence also supports the utility of PET-CT in treatment response assessment, facilitating timely optimization of appropriate treatment regimens (1112). Magnetic resonance imaging (MRI) is employed in the diagnosis and follow-up of extranodal NK/T-cell lymphoma and subcutaneous panniculitis like T-cell lymphoma, due to superior soft tissue contrast in the nasal cavity and subcutaneous tissues, respectively (1314). The purpose of this review is to familiarize radiologists with clinical features and imaging manifestations of T-cell NHL as well as the role of CT, PET-CT and MRI in the management of common subtypes.
Mycosis fungoides (MF) and Sézary syndrome (SS) are the most common subtypes of CTCL, which account for approximately 6% of NHLs (515). These entities present with skin involvement and are to be distinguished from other T-cell lymphomas which may involve the skin, to be discussed later (16). MF is generally indolent, and diagnosis is based upon clinical findings of erythematous patches/plaques often originating on the trunk as well as histopathologic findings of neoplastic CD3+ and CD4+ T-cells in the skin (17). Affected patients may have had several years of skin lesions confused with benign entities, even on biopsy due to accompanying non-malignant inflammatory infiltrates. Multiple skin biospies may be necessary for diagnosis. Skin lesions progress from patches to plaques to cutaneous tumors. SS may evolve from MF or can present de novo, characterized by skin rash and malignant lymphocytes within the peripheral blood (Sezary cells) (16). Extensive skin involvement, peripheral lymphadenopathy and visceral disease may be present. In contrast to MF, SS is more symptomatic and is associated with lower remission and survival rates.
In MF, patients with only cutaneous lesions including erythema, patches, and/or plaques may demonstrate no imaging/CT abnormalities. If the disease progresses to form a discrete mass, then skin thickening or a mass is seen on CT if one is performed (18). In the context of clinical trials, CT is advised when it is important to fully stage participants and have the ability to assess response (19). Repeat examinations may not be needed in patients with clinically early disease, while follow-up imaging is useful in advanced disease to detect response to treatment and progression, especially in terms of nodal or visceral involvement. PET-CT is not routinely performed in MF/SS, though it has been shown more sensitive than CT in detecting nodal involvement and defining reactive versus malignant nodes (91820). In one report, PET-CT was shown useful for more accurate measurement of skin lesion thickness and nodal staging, both of which have been correlated to outcomes (Fig. 1) (18). Imaging contributes to accurate staging in advanced CTCL, whereby tumor lesions indicate stage IIB, erythroderma in the absence of nodal or visceral disease indicates stage III, histologic lymph node involvement, irrespective of T stage represents stage IVA, and visceral disease represents stage IVB, irrespective of T and N stage. It is important to detect lymphadenopathy and/or other visceral organ involvement since survival rates decrease dramatically with visceral involvement. Pulmonary involvement is the most common extra-nodal involvement of CTCL, associated with poor outcomes (2122).
Several long-term prognostic studies have revealed that patients with early CTCL have a favorable prognosis. In fact, patients with stage IA disease may have complete resolution of skin lesions with treatment, which may include psoralen and ultraviolet A radiation, electron-beam radiation, topical and other treatments (16). However, patients with advanced CTCL have a poor prognosis despite intensified therapy including systemic chemotherapy. The extent of cutaneous involvement (ranging from T1–T4) is significantly associated with prognosis and disease progression. One large study found that the risk for disease progression at 5 years was 10% in T1, 22% in T2, and 48% to 56% in T3 to T4 levels of cutaneous involvement (23). For treatment response assessment, PET-CT can be used to monitor lymph nodes that are 18FDG-avid but do not meet the size criteria for staging (182425). In addition, changes in 18FDG uptake in the form of maximum standardized uptake values (SUVmax) can potentially impact patient management (18).
At present, CTCL treatment is not based on specific genetic or molecular targets. However, altered molecular pathways and genetic drivers are being discovered and studied, to better understand the pathogenesis of this disease and to identify potential targets for treatment, including mutations in the T-cell receptor complex, nuclear factor-κB, and the Janus kinase/signal transducer and activator of transcription signaling pathways (26). Biomarkers are sought to guide management in targeted and non-targeted treatment settings.
Mycosis fungoides may undergo a process of large cell transformation (LCT), which is characterized by more aggressive disease, in up to 39% of MF patients (Fig. 2) (2728). LCT is defined histopathologically by the presence of large cells that are at least 4 times the size of lymphocytes exceeding 25% of the cell populations (29). LCT can occur in any stage of CTCL, however, it more frequently occurs in advanced stages. LCT is commonly found in skin tumors but may occur at any involved site, such as nodes (29). PET-CT may be useful in patients with suspected LCT. According to Feeney et al. (9), average SUVmax of cutaneous lesions in LCT is significantly higher compared to those in MF or SS without LCT. The prognosis of LCT is significantly worse than MF. In one report, median survival of LCT patients from initial diagnosis of MF/SS was 37 months, compared with 163 months in patients with no transformation (30). It is recommended that any patient with MF who develops new papules receive a biopsy to evaluate for LCT and subsequent PET-CT. If LCT is confirmed, patients are treated more aggressively, with systemic chemotherapy and possibly autologous or allogeneic hematopoietic cell transplantation (HCT) and/or radiation (29).
Peripheral T-cell lymphoma, NOS is a heterogeneous group of predominantly nodal T-cell lymphomas. It is the most common subtype of PTCL, accounting for approximately 30% of PTCL and approximately 4% of NHLs overall (5). PTCL NOS is usually aggressive and relapse is common. Most patients present with generalized lymphadenopathy with or without extra-nodal disease, including involvement of skin, gastrointestinal tract, liver, spleen or marrow (3132333435). Approximately 60% of patients with PTCL NOS have stage IV disease at the time of diagnosis (35). Diagnosis of PTCL NOS is made based upon the results of a tissue biopsy, usually of a lymph node. However, because PTCL NOS is essentially a diagnosis of exclusion, the diagnostic agreement rate among expert hematopathologists in one report was only about 75% (33). Gene expression profiling studies have identified subtypes of PTCL NOS with differing clinical behavior and response to therapy (236).
On CT, generalized lymphadenopathy is the most common finding in PTCL NOS. The imaging appearance is generally not distinguishable from other types of lymphoma. On PET-CT, PTCL NOS is often 18FDG-avid, as seen in 33 of 34 patients in one report with SUVmax ranging from 2.8 to 42.3 (mean 12.3) (Fig. 3) (9). According to the Lugano classification guidelines, which are the most recent recommendations for initial evaluation, staging, and response assessment of Hodgkin and NHL, PET-CT is recommended for routine staging of 18FDG-avid, nodal lymphomas (37). It may become more commonly used in PTCL NOS.
The standard treatment for PTCL NOS is a conventional-dose systemic anthracycline-containing chemotherapy (3). The role of HCT is still investigational and the potential benefit of HCT is difficult to ascertain due to reports combining PTCL subtypes and disease rarity (38). Poor prognostic factors in PTCL NOS include age > 60, performance status ≥ 2, elevated lactate dehydrogenase (LDH) and bone marrow involvement (39). According to one multicenter study, the cumulative 5-year overall survival of PTCL NOS was 43% (39). Attempts have been made to identify biologically and prognostically distinct subgroups within the heterogeneous PTCL NOS category. For example, Epstein-Barr virus (EBV) is found in approximately 30% of all cases of PTCL NOS and may be associated with a more aggressive course (40).
Angioimmunoblastic T-cell lymphoma (AITL) is a rare subtype of PTCL, which accounts for 1–2% of NHLs and 15–20% of PTCLs (3). It is more common in elderly, and patients often present with an acute onset systemic illness including B symptoms, generalized lymphadenopathy, and hepatosplenomegaly at the time of diagnosis. The CT features are nonspecific and similar to those of any disseminated lymphoma. The lesions usually exhibit high 18FDG avidity (Fig. 4) (41). AITL is generally an aggressive disease, although occasional spontaneous remissions are seen. Relapse is frequent and overall survival is low, reportedly 32% in one report (42).
In the WHO classification, systemic anaplastic large cell lymphoma (ALCL) can be divided into two subgroups on the basis of the expression of anaplastic lymphoma kinase (ALK) protein. ALK-positive ALCL is characterized by a chromosomal translocation linking the ALK kinase gene on chromosome 2 to the nucleophosmin gene on chromosome 5, resulting in a constitutively active tyrosine kinase (43). The distinction of ALK-positive and ALK-negative ALCL is important because of clinical and prognostic differences. Namely, ALK-positive ALCL is more common in younger patients who generally have superior outcomes when treated with standard chemotherapy as compared to patients with ALK-negative ALCL (43). ALK-positive ALCL patients typically have 5-year overall survival rates of more than 70%, whereas patients with ALK-negative ALCL have 5-year overall survival rates less than 50% (4344).
Patients with ALCL often present with painless lymphadenopathy, with or without extra-nodal involvement (Fig. 5). One study has shown differences in extra-nodal sites involved in ALK-positive and ALK-negative ALCLs: bone marrow, bone, subcutaneous tissue, and splenic involvement were seen at more commonly in ALK-positive patients, whereas skin, liver, and gastrointestinal involvement was more frequent in ALK-negative ALCL (43). The degree of 18FDG uptake is higher in ALK-positive ALCL than in ALK-negative ALCL, suggesting that PET may be useful in distinguishing between the two groups (45).
Extranodal NK/T-cell lymphoma is most common in Asia and in native populations of Central and South America, and it is rare in the United States and Europe (346). It is strongly associated with EBV infection. 60–80% patients present with localized disease involving aerodigestive tract, resulting in symptoms of nasal obstruction, epistaxis and/or destructive mass involving the nasal cavity, sinuses or oral cavity (47). Lymph nodes may be involved secondarily but are rarely the primary site of involvement. Extranasal involvement is common in soft tissues/skin, the gastrointestinal tract, lungs, adrenal glands, testes or central nervous system (CNS) (47). Extranasal extranodal NK/T-cell lymphoma is associated with more aggressive features in terms of advanced stage at presentation, poorer performance status of affected patients, inferior response to chemotherapy and poorer survival (4748).
On CT, tumors show soft tissue attenuation with mild to moderate heterogeneous enhancement. MRI is better demonstrating the extent of lesion as compared to CT, especially in the nasal type (Fig. 6) (49). Signal intensity of tumors is higher than that of muscle but lower than that of sinonasal mucosa on T2-weighted spin-echo images. On T1-weighted spin-echo images, tumors show similar to higher signal intensity than that of muscle. PET-CT is a useful tool for initial diagnosis and staging, because both nasal and extranasal lesions exhibit high 18FDG avidity (Fig. 7) (415051). Radiotherapy has been validated as primary treatment for localized nasal NK/T-cell lymphoma in several large retrospective studies (5253545556). As expected, patients with early stage disease have a better prognosis as compared to patients with advanced disease.
Subcutaneous panniculitis-like T-cell lymphoma (SPTCL) is a rare subtype of peripheral T-cell lymphoma, accounting for less than 1 percent of all NHLs (3). Patients with SPTCL typically present with one or more usually painless nodules or poorly circumscribed indurated plaques most commonly located on the trunk or lower extremities (5758). Initial diagnosis of SPTCL is often delayed because inflammatory or infectious processes may be suspected both clinically and on imaging. MRI features of SPTCL include nodular or mass-like enhancing areas that infiltrate the subcutaneous tissues (Fig. 8) (14). Central areas of intermediate T2-weighted signal intensity (compared to muscle) may be seen, which can be useful in differentiating SPTCL from other inflammatory or infectious processes (14). Both CT and PET-CT are useful for recognizing other organ involvement and evaluating the extent of disease (4059). Whole body MRI has also been suggested as a useful tool at initial diagnosis and follow-up, to assess the extent of disease and monitor a patient's response to therapy (60). The clinical course of SPTCL can be indolent with relapsing panniculitis, or aggressive with the development of a hemophagocytic syndrome which can be fatal. Favorable (αβ) and poor (γδ) prognostic phenotypes have been described (57).
Precursor T-cell lymphoblastic lymphoma is a rare but highly aggressive neoplasm of lymphoblasts of T-cell origin, with an incidence of approximately 2% of adult NHLs. Patients are commonly young adults who may present with lymphadenopathy in the cervical, supraclavicular, and axillary regions and/or bulky mediastinal masses (Fig. 9) (616263). Superior vena cava syndrome, tracheal obstruction and pleural and/or pericardial effusions may be associated (64). Extranodal disease (e.g., involvement of the skin, testis, liver, spleen and/or bone) is a less common presentation. Central nervous system involvement more commonly occurs in patients with bone marrow involvement.
Imaging studies such as total body CT scan (head and neck, thorax, abdomen, and pelvis) and PET-CT are used for staging and therapeutic response evaluation. For post induction response assessment, CT scan is useful for evaluation of intrathoracic and abdominal disease involvement. Mediastinal mass assessment is important in T-LBL, as residual masses can remain after treatment and mediastinal relapses can occur. Most T-LBL exhibit high 18FDG avidity on PET-CT at baseline, prior to the start of treatment (9406264). Although PET did not predict long-term outcome in previous retrospective studies (6566), PET-negativity is useful because it may eliminate the need for intensification of chemotherapy or mediastinal irradiation.
A variety of therapeutic approaches in T-LBL have been reported, including chemotherapy protocols for high grade NHL and HCT. Complete response rates of greater than 80% and a disease-free survival rate of 56% have been reported in adults treated with intensive/acute lymphoblastic leukemia-type regimens (616768). While prognostic models are not yet defined for adult T-LBL, poor risk features in adults include age > 30 years, advanced stage III or IV disease, high LDH level (more than 1.5 times normal), involvement of central nervous system, bone marrow or mediastinum (676970). Patients with poor risk features are potential candidates for intensified therapy and HCT (1468). Song et al. (71) achieved higher event free survival and overall survival in T-LBL patients who proceeded to HCT compared to patients treated with chemotherapy alone. Disease relapse has been reported as a leading cause of death after HCT, followed by infections, graft-versus-host disease, respiratory, cardiovascular and other treatment-associated complications (Fig. 10) (7273).
T-cell NHLs include a wide spectrum of diseases ranging from indolent to highly aggressive, associated with varied clinical features and prognosis. It is important to have familiarity with common subtypes of T-cell NHL, including the expected clinical courses and prognostic factors, to best interpret imaging studies and guide management in affected patients. Specifically, cutaneous disease is often indolent; nodal and/or visceral involvement indicates the need for more aggressive treatment. PTCL NOS is a common subtype, often aggressive. Precursor T-LBL is aggressive, with poor prognosis. Imaging studies including CT, MRI and PET-CT have important and specific roles in the various entities, not only for staging but also in response assessment and detection of disease-related and treatment-related complications.
Figures and Tables
Fig. 1
18FDG PET-CT in patient with cutaneous T-cell lymphoma with treatment response.
Baseline (A) and post treatment (B) axial fused PET-CT images demonstrate abnormal skin thickening in right posterior upper thigh/inferior buttock with associated intense 18FDG uptake (arrows), which resolved after treatment with radiation, photochemotherapy, and topical steroids. FDG = flourodeoxyglucose, PET = positron emission tomography
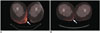
Fig. 2
Contrast-enhanced CT and 18FDG PET-CT in patient with transformed cutaneous T-cell lymphoma.
Axial contrast-enhanced CT image (A) demonstrates diffuse skin thickening in left groin (arrows), which resolved following treatment with radiation, phototherapy, and Targretin (not shown). Unfortunately, on subsequent restaging contrast-enhanced chest CT (B), patient developed bilateral pulmonary masses (arrows), which were biopsy proven cutaneous T-cell lymphoma. As seen on axial fused PET-CT image (C), pulmonary lesions increased in size and number despite treatment with cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) regimen (arrow), pralatrexate, gemcitabine, Ontak, allogenic stem cell transplant, and palliative radiation and patient eventually expired from pulmonary complications. FDG = flourodeoxyglucose, PET = positron emission tomography

Fig. 3
18FDG PET-CT in patient with peripheral T-cell lymphoma, not otherwise specified with treatment response.
Baseline MIP (A) and axial fusion (B) PET-CT images demonstrate intense 18FDG uptake in left neck corresponding with large soft tissue mass and intense 18FDG uptake in left base of tongue (arrows). Additional sites of 18FDG uptake are seen in upper paratracheal region, inguinal stations, and right thigh (arrowheads). Post treatment MIP (C) and axial fusion (D) images following treatment with RCHOP and neck radiation demonstrate resolution of 18FDG uptake and soft tissue mass. FDG = flourodeoxyglucose, MIP = maximum intensity projection, PET = positron emission tomography
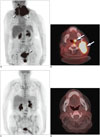
Fig. 4
18FDG PET-CT and diagnostic contrast-enhanced chest CT in patient with angioimmunoblastic T-cell lymphoma.
MIP image (A) demonstrates intense 18FDG uptake in cervical, thoracic, abdominal, and left inguinal lymph node stations, subcutaneous nodules, and bilateral pulmonary nodules, masses, and consolidations. Focal 18FDG uptakes in left arm are likely related to injection. Axial fused PET-CT image (B) demonstrates intense 18FDG uptake right axillary lymphadenopathy (arrow) and within bilateral pulmonary nodules and masses. Axial contrast-enhanced CT image on lung window (C) demonstrates pulmonary nodules and masses with surrounding groundglass opacities and mild intralobular septal thickening. One of lung masses was biopsied, and proven to be angioimmunoblastic lymphoma. Patient had complete response after treatment with CHOP and underwent autologous stem cell transplant but developed myelodysplastic syndrome and T-cell lymphoma recurrence and expired. FDG = flourodeoxyglucose, MIP = maximum intensity projection, PET = positron emission tomography
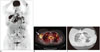
Fig. 5
18FDG PET-CT in patient with anaplastic large cell lymphoma with treatment response.
Baseline CT (A) and PET (B) images demonstrate intense 18FDG uptake within left external iliac and pelvic sidewall lymphadenopathy (arrows). Post treatment CT (C) and PET (D) images demonstrate complete resolution of lymphadenopathy and 18FDG uptake after treatment with CHOP. FDG = flourodeoxyglucose, PET = positron emission tomography
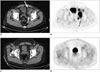
Fig. 6
MRI and 18FDG PET-CT in patient with extranodal natural killer T-cell lymphoma of nasal cavity.
Axial T2-weighted MR image (A) shows diffuse thickening of right nasal mucosa with T2-hypointensity (arrows) compared to left side. Axial fused PET-CT image (B) demonstrates corresponding intense 18FDG uptake in right nasal cavity (arrows). FDG = flourodeoxyglucose, PET = positron emission tomography
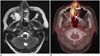
Fig. 7
18FDG PET-CT in patient with extranodal natural killer T-cell lymphoma.
MIP (A) and axial fused PET-CT (B) images demonstrate intense 18FDG uptake in left lower extremity, involving marrow compartment of distal left tibia and surrounding subcutaneous soft tissue and overlying skin medially (arrows). FDG = flourodeoxyglucose, MIP = maximum intensity projection, PET = positron emission tomography
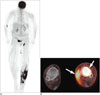
Fig. 8
Contrast-enhanced MRI in patient with subcutaneous panniculitis-like T-cell lymphoma.
Axial T2 fat-sat (A) and T1-weighted (B) images demonstrate vague areas of increased T2 hyperintensity and T1 isointensity in subcutaneous tissues of posterior proximal thighs (arrows). Axial T1-weighted postcontrast (C) image demonstrates corresponding areas of enhancement (arrows).
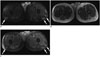
Fig. 9
18FDG PET-CT images in patient with precursor T-cell lymphoma with treatment response.
Baseline axial fused PET-CT image (A) demonstrates large hypo to isodense anterior mediastinal mass with mild to moderate 18FDG uptake (arrows). Post treatment axial fused PET-CT image (B) show resolution of anterior mediastinal mass and abnormal 18FDG uptake following treatment with Larson protocol intensification. FDG = flourodeoxyglucose, PET = positron emission tomography
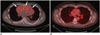
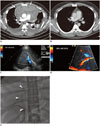
Fig. 10
Contrast-enhanced chest CT in patient with precursor T-cell lymphoma with surgical resection.
Baseline axial CT image (A) demonstrates large heterogeneously enhancing anterior mediastinal mass with associated pericardial effusion (arrows) and bilateral pleural effusions. Patient was taken for urgent pericardial window procedure, biopsy, which was initially thought to represent thymoma. Patient was treated with urgent radiation, chemotherapy with cytoxan, adriamycin, and cisplatin, and eventual surgical resection showing no residual disease in specimen, which was thought to be unusual for thymoma. Postsurgical axial CT image (B) demonstrates mild stranding in anterior mediastinum, consistent with postsurgical changes, without evidence for residual or recurrent lymphoma. Re-review of initial biopsy was felt to represent precursor T-cell lymphoma. Patient underwent induction chemotherapy per CALGB 9111 protocol and stem cell transplant. Following stem cell transplant, patient unfortunately developed veno-occlusive disease, as seen on ultrasound color Doppler images showing reversal of flow in right (C) and main (D) portal veins and expired despite transjugular intrahepatic portosystemic shunt procedure (arrowheads) (E).
References
1. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997; 89:3909–3918.
2. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127:2375–2390.
3. Vose J, Armitage J, Weisenburger D. International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008; 26:4124–4130.
4. Abouyabis AN, Shenoy PJ, Lechowicz MJ, Flowers CR. Incidence and outcomes of the peripheral T-cell lymphoma subtypes in the United States. Leuk Lymphoma. 2008; 49:2099–2107.
5. Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006; 107:265–276.
6. Vinnicombe SJ, Reznek RH. Computerised tomography in the staging of Hodgkin's disease and non-Hodgkin's lymphoma. Eur J Nucl Med Mol Imaging. 2003; 30:Suppl 1. S42–S55.
7. Raanani P, Shasha Y, Perry C, Metser U, Naparstek E, Apter S, et al. Is CT scan still necessary for staging in Hodgkin and non-Hodgkin lymphoma patients in the PET/CT era? Ann Oncol. 2006; 17:117–122.
8. Cheson BD. Role of functional imaging in the management of lymphoma. J Clin Oncol. 2011; 29:1844–1854.
9. Feeney J, Horwitz S, Gönen M, Schöder H. Characterization of T-cell lymphomas by FDG PET/CT. AJR Am J Roentgenol. 2010; 195:333–340.
10. Tatsumi M, Cohade C, Nakamoto Y, Fishman EK, Wahl RL. Direct comparison of FDG PET and CT findings in patients with lymphoma: initial experience. Radiology. 2005; 237:1038–1045.
11. Cronin CG, Swords R, Truong MT, Viswanathan C, Rohren E, Giles FJ, et al. Clinical utility of PET/CT in lymphoma. AJR Am J Roentgenol. 2010; 194:W91–W103.
12. Moskowitz CH, Schöder H. Current status of the role of PET imaging in diffuse large B-cell lymphoma. Semin Hematol. 2015; 52:138–142.
13. Gill H, Liang RH, Tse E. Extranodal natural-killer/T-cell lymphoma, nasal type. Adv Hematol. 2010; 2010:627401.
14. Levine BD, Seeger LL, James AW, Motamedi K. Subcutaneous panniculitis-like T-cell lymphoma: MRI features and literature review. Skeletal Radiol. 2014; 43:1307–1311.
15. Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007; 143:854–859.
16. National Cancer Institute. PDQ® adult treatment editorial board. PDQ mycosis fungoides and the Sézary syndrome treatment. Web site. Accessed June 28, 2016. http://www.cancer.gov/types/lymphoma/hp/mycosis-fungoides-treatment-pdq.
17. Siegel RS, Pandolfino T, Guitart J, Rosen S, Kuzel TM. Primary cutaneous T-cell lymphoma: review and current concepts. J Clin Oncol. 2000; 18:2908–2925.
18. Kuo PH, McClennan BL, Carlson K, Wilson LD, Edelson RL, Heald PW, et al. FDG-PET/CT in the evaluation of cutaneous T-cell lymphoma. Mol Imaging Biol. 2008; 10:74–81.
19. Olsen EA, Whittaker S, Kim YH, Duvic M, Prince HM, Lessin SR, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011; 29:2598–2607.
20. Jhanwar YS, Straus DJ. The role of PET in lymphoma. J Nucl Med. 2006; 47:1326–1334.
21. Ueda T, Hosoki N, Isobe K, Yamamoto S, Motoori K, Shinkai H, et al. Diffuse pulmonary involvement by mycosis fungoides: high-resolution computed tomography and pathologic findings. J Thorac Imaging. 2002; 17:157–159.
22. Baser S, Onn A, Lin E, Morice RC, Duvic M. Pulmonary manifestations in patients with cutaneous T-cell lymphomas. Cancer. 2007; 109:1550–1555.
23. Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003; 139:857–866.
24. Spaccarelli N, Gharavi M, Saboury B, Cheng G, Rook AH, Alavi A. Role of (18)F-fluorodeoxyglucose positron emission tomography imaging in the management of primary cutaneous lymphomas. Hell J Nucl Med. 2014; 17:78–84.
25. Kim JS, Jeong YJ, Sohn MH, Lim ST, Kim DW, Jeong HJ, et al. Before and after treatment 18F-FDG PET/CT images in a patient with cutaneous T-cell lymphoma. Eur J Nucl Med Mol Imaging. 2010; 37:1995.
26. Damsky WE, Choi J. Genetics of cutaneous T cell Lymphoma: from bench to bedside. Curr Treat Options Oncol. 2016; 17:33.
27. Vergier B, de Muret A, Beylot-Barry M, Vaillant L, Ekouevi D, Chene G, et al. French Study Group of Cutaneious Lymphomas. Transformation of mycosis fungoides: clinicopathological and prognostic features of 45 cases. Blood. 2000; 95:2212–2218.
28. Salhany KE, Cousar JB, Greer JP, Casey TT, Fields JP, Collins RD. Transformation of cutaneous T cell lymphoma to large cell lymphoma. A clinicopathologic and immunologic study. Am J Pathol. 1988; 132:265–277.
29. Herrmann JL, Hughey LC. Recognizing large-cell transformation of mycosis fungoides. J Am Acad Dermatol. 2012; 67:665–672.
30. Diamandidou E, Colome-Grimmer M, Fayad L, Duvic M, Kurzrock R. Transformation of mycosis fungoides/Sezary syndrome: clinical characteristics and prognosis. Blood. 1998; 92:1150–1159.
31. Chott A, Dragosics B, Radaszkiewicz T. Peripheral T-cell lymphomas of the intestine. Am J Pathol. 1992; 141:1361–1371.
32. Bekkenk MW, Vermeer MH, Jansen PM, van Marion AM, Canninga-van Dijk MR, Kluin PM, et al. Peripheral T-cell lymphomas unspecified presenting in the skin: analysis of prognostic factors in a group of 82 patients. Blood. 2003; 102:2213–2219.
33. Weisenburger DD, Savage KJ, Harris NL, Gascoyne RD, Jaffe ES, MacLennan KA, et al. Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood. 2011; 117:3402–3408.
34. Savage KJ, Ferreri AJ, Zinzani PL, Pileri SA. Peripheral T-cell lymphoma--not otherwise specified. Crit Rev Oncol Hematol. 2011; 79:321–329.
35. Vose JM. Peripheral T-cell non-Hodgkin's lymphoma. Hematol Oncol Clin North Am. 2008; 22:997–1005. x
36. Wang T, Feldman AL, Wada DA, Lu Y, Polk A, Briski R, et al. GATA-3 expression identifies a high-risk subset of PTCL, NOS with distinct molecular and clinical features. Blood. 2014; 123:3007–3015.
37. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32:3059–3068.
38. Shustov AR, Savage KJ. Does high-dose therapy and autologous hematopoietic stem cell transplantation have a role in the primary treatment of peripheral T-cell lymphomas? ASH evidence-based review 2008. Hematology Am Soc Hematol Educ Program. 2008; 39–41.
39. Gallamini A, Stelitano C, Calvi R, Bellei M, Mattei D, Vitolo U, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004; 103:2474–2479.
40. Dupuis J, Emile JF, Mounier N, Gisselbrecht C, Martin-Garcia N, Petrella T, et al. Prognostic significance of Epstein-Barr virus in nodal peripheral T-cell lymphoma, unspecified: A Groupe d'Etude des Lymphomes de l'Adulte (GELA) study. Blood. 2006; 108:4163–4169.
41. Weiler-Sagie M, Bushelev O, Epelbaum R, Dann EJ, Haim N, Avivi I, et al. (18)F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med. 2010; 51:25–30.
42. Federico M, Rudiger T, Bellei M, Nathwani BN, Luminari S, Coiffier B, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013; 31:240–246.
43. Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008; 111:5496–5504.
44. Parrilla Castellar ER, Jaffe ES, Said JW, Swerdlow SH, Ketterling RP, Knudson RA, et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood. 2014; 124:1473–1480.
45. Lee DY, Lee JJ, Kim JY, Park SH, Chae SY, Kim S, et al. (18)F-FDG PET in patients with primary systemic anaplastic large cell lymphoma: differential features according to expression of anaplastic lymphoma kinase. Nucl Med Mol Imaging. 2013; 47:249–256.
46. Panwalkar AW, Armitage JO. T-cell/NK-cell lymphomas: a review. Cancer Lett. 2007; 253:1–13.
47. Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009; 113:3931–3937.
48. Ahn HK, Suh C, Chuang SS, Suzumiya J, Ko YH, Kim SJ, et al. Extranodal natural killer/T-cell lymphoma from skin or soft tissue: suggestion of treatment from multinational retrospective analysis. Ann Oncol. 2012; 23:2703–2707.
49. Ou CH, Chen CC, Ling JC, Chai JW, Wu CH, Chen WH, et al. Nasal NK/T-cell lymphoma: computed tomography and magnetic resonance imaging findings. J Chin Med Assoc. 2007; 70:207–212.
50. Zhou X, Lu K, Geng L, Li X, Jiang Y, Wang X. Utility of PET/CT in the diagnosis and staging of extranodal natural killer/T-cell lymphoma: a systematic review and meta-analysis. Medicine (Baltimore). 2014; 93:e258.
51. Wu HB, Wang QS, Wang MF, Li HS, Zhou WL, Ye XH, et al. Utility of 18F-FDG PET/CT for staging NK/T-cell lymphomas. Nucl Med Commun. 2010; 31:195–200.
52. Li YX, Fang H, Liu QF, Lu J, Qi SN, Wang H, et al. Clinical features and treatment outcome of nasal-type NK/T-cell lymphoma of Waldeyer ring. Blood. 2008; 112:3057–3064.
53. Li YX, Yao B, Jin J, Wang WH, Liu YP, Song YW, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006; 24:181–189.
54. Pan ZH, Huang HQ, Lin XB, Xia YF, Xia ZJ, Peng YL, et al. [Prognostic analysis of patients with nasal-type NK/T-cell non-Hodgkin's lymphoma--a report of 93 cases]. Ai Zheng. 2005; 24:1493–1497.
55. Kim GE, Lee SW, Chang SK, Park HC, Pyo HR, Kim JH, et al. Combined chemotherapy and radiation versus radiation alone in the management of localized angiocentric lymphoma of the head and neck. Radiother Oncol. 2001; 61:261–269.
56. Li CC, Tien HF, Tang JL, Yao M, Chen YC, Su IJ, et al. Treatment outcome and pattern of failure in 77 patients with sinonasal natural killer/T-cell or T-cell lymphoma. Cancer. 2004; 100:366–375.
57. Willemze R, Jansen PM, Cerroni L, Berti E, Santucci M, Assaf C, et al. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008; 111:838–845.
58. Jeong SI, Lim HS, Choi YR, Kim JW, Park MH, Cho JS, et al. Subcutaneous panniculitis-like T-cell lymphoma of the breast. Korean J Radiol. 2013; 14:391–394.
59. Papajík T, Mysliveček M, Sedová Z, Buriánková E, Procházka V, Koranda P, et al. Standardised uptake value of 18F-FDG on staging PET/CT in newly diagnosed patients with different subtypes of non-Hodgkin’s lymphoma. Eur J Haematol. 2011; 86:32–37.
60. Lim GY, Hahn ST, Chung NG, Kim HK. Subcutaneous panniculitis-like T-cell lymphoma in a child: whole-body MRI in the initial and follow-up evaluations. Pediatr Radiol. 2009; 39:57–61.
61. Hoelzer D, Gökbuget N, Digel W, Faak T, Kneba M, Reutzel R, et al. Outcome of adult patients with T-lymphoblastic lymphoma treated according to protocols for acute lymphoblastic leukemia. Blood. 2002; 99:4379–4385.
62. Otero HJ, Jagannathan JP, Prevedello LM, Johnston CJ, Ramaiya NH, Van den Abbeele AD, et al. CT and PET/CT findings of T-cell lymphoma. AJR Am J Roentgenol. 2009; 193:349–358.
63. Morel P, Lepage E, Brice P, Dupriez B, D'Agay MF, Fenaux P, et al. Prognosis and treatment of lymphoblastic lymphoma in adults: a report on 80 patients. J Clin Oncol. 1992; 10:1078–1085.
64. Cortelazzo S, Ponzoni M, Ferreri AJ, Hoelzer D. Lymphoblastic lymphoma. Crit Rev Oncol Hematol. 2011; 79:330–343.
65. Gökbuget N, Wolf A, Stelljes M, Hüttmann A, Buss EC, Viardot A, et al. Favorable outcome in a large cohort of prospectively treated adult patients with T-lmphoblastic lymphoma (T-LBL) despite slowly evolving complete remission assessed by conventional radiography. Blood. 2014; 124:370.
66. Lepretre S, Touzart A, Vermeulin T, Picquenot JM, Tanguy-Schmidt A, Salles G, et al. Pediatric-like acute lymphoblastic leukemia therapy in adults with lymphoblastic lymphoma: the GRAALL-LYSA LL03 study. J Clin Oncol. 2016; 34:572–580.
67. Aljurf M, Zaidi SZ. Chemotherapy and hematopoietic stem cell transplantation for adult T-cell lymphoblastic lymphoma: current status and controversies. Biol Blood Marrow Transplant. 2005; 11:739–754.
68. Kaiser U, Uebelacker I, Havemann K. Non-Hodgkin's lymphoma protocols in the treatment of patients with Burkitt's lymphoma and lymphoblastic lymphoma: a report on 58 patients. Leuk Lymphoma. 1999; 36:101–108.
69. Coleman CN, Picozzi VJ Jr, Cox RS, McWhirter K, Weiss LM, Cohen JR, et al. Treatment of lymphoblastic lymphoma in adults. J Clin Oncol. 1986; 4:1628–1637.
70. Slater DE, Mertelsmann R, Koziner B, Higgins C, McKenzie S, Schauer P, et al. Lymphoblastic lymphoma in adults. J Clin Oncol. 1986; 4:57–67.
71. Song KW, Barnett MJ, Gascoyne RD, Chhanabhai M, Forrest DL, Hogge DE, et al. Primary therapy for adults with T-cell lymphoblastic lymphoma with hematopoietic stem-cell transplantation results in favorable outcomes. Ann Oncol. 2007; 18:535–540.
72. Mohty B, Mohty M. Long-term complications and side effects after allogeneic hematopoietic stem cell transplantation: an update. Blood Cancer J. 2011; 1:e16.
73. Afessa B, Peters SG. Major complications following hematopoietic stem cell transplantation. Semin Respir Crit Care Med. 2006; 27:297–309.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download