Abstract
Yolk sac tumor (YST) is a rare germ cell neoplasm of childhood that usually arises from the testis or ovary. The rare cases of YST in various extragonadal locations have been reported, but the primary intrarenal YST is even more uncommon. Here, we report a case of a primary intrarenal YST with tumor thrombus of the inferior vena cava and left renal vein in a 2-year-old boy, with an emphasis on the CT features. To our knowledge, this is the first reported case of an intrarenal YST with intravascular involvement.
Pediatric malignant germ cell tumors are the uncommon neoplasms of childhood, accounting for only 3% of the pediatric malignancies (1). Yolk sac tumor (YST), also referred to as endodermal sinus tumor, is a rare germ cell neoplasm occurring primarily in the gonads. The primary extragonadal YSTs are exceedingly rare, which occur among 10% to 15% of these patients (2). The most common extragonadal sites are the mediastinum, vagina, brain, and retroperitoneum. Other cases of YST at rare sites such as prostate, omentum, liver, and lung have also been reported (2, 3). Only three intrarenal cases of YST have been reported in the medical literature in English (3, 4, 5). In these cases, a tumor extension into the inferior vena cava (IVC) and left renal vein has not been described. To the best of our knowledge, this is the first reported case of intrarenal YST with tumor thrombus of the IVC and left renal vein.
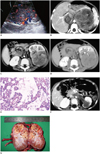
A 2-year-old boy was admitted to our hospital with a gross hematuria after trauma, which had begun 7 days earlier. The abdominal ultrasound with color Doppler study showed a large, complex mass with increased vascularity in the left kidney, which extended into the IVC (Fig. 1A). The patient underwent both pre- and post-contrast CT examination. There were three dynamic contrast-enhanced phases, including cortical phase, corticomedullary phase, and excretory phase. The scan parameters used for the Toshiba Xpress/SX spiral CT scanner were as follows: 120 kVp, 200 mAs, 1 second scan time, and 5 mm slice thickness. CT dose index (CTDI) was estimated to be 60 mGy. The CTDI body phantom (32 cm) was used for the calculation of CTDI in this CT examination. The contrast-enhanced abdominal CT scan showed a heterogeneous, enhancing mass in the upper pole of the left kidney with multiple enlarged vessels in the mass (Fig. 1B). The mass was poorly defined with fascial thickening, which represents tumor extension into the perirenal space. The multiple small nodules within the tumor showed ring-shaped enhancement with central low density area (Fig. 1C). A tumor thrombus in the IVC measuring 7 × 3 × 3 cm was also noted (Fig. 1B), and its enhancement was the same as that of the renal mass. The left renal vein could not be identified due to the compression by the huge mass. The tumor nodules encased the pelvocaliceal system, but hydronephrosis was not observed (Fig. 1D). No evidence of intratumoral calcifications was seen. The paraaortic and pararenal lymphadenopathy and a small amount of ascites around the spleen were present. The imaging features of the mass were highly suggestive of Wilms tumor. However, laboratory tests revealed markedly elevated serum level of α-fetoprotein (AFP, 60342.5 ng/mL; nomal value, less than 20 ng/mL). The ultrasonography-guided fine-needle aspiration was performed. The cytological examination of the mass revealed numerous Schiller-Duval bodies, which are the typical patterns of YST (Fig. 1E). Subsequently, testicular ultrasound was performed and no abnormality was found.
Chemotherapy with vincristine and actinomycin D was started on the patient. After the three courses of chemotherapy, follow-up CT scan showed a significant decrease in the size of the left renal mass and IVC thrombus. The tumor thrombus of the left renal vein was identified clearly (Fig. 1F). The serum AFP level declined to 805.8 ng/mL. Then, the left radical nephrectomy with IVC and the left renal vein thrombectomy were carried out. A 5 × 4 × 3 cm solid mass was found in the upper pole of the left kidney. Palpation of the left renal vein and IVC showed firmness in their anterior walls. No other abnormal findings were identified.
The cut surface of the mass was solid and variegated. The mass contained multiple, rounded tumor lobules with central hemorrhage (Fig. 1G). Infiltration of the collecting system was noted. Microscopically, the neoplasm showed granulation and fibrosis with foci of tumor cells, which is suggestive of partial response to chemotherapy.
Three weeks after the surgery, the patient was restarted on neoadjuvant chemotherapy with vincristine, actinomycin D, and cisplatinum or cyclophosphamide. Serum AFP level had fallen to the normal range after three courses. The patient was followed up for 13 months without any clinical and radiologic evidence of recurrence.
Yolk sac tumor can occur anywhere, although it usually occurs in the gonads (2, 3). The most common extragonadal sites are in the midline, particularly in the retroperitoneum, anterior mediastinum, sacrococcygeal region, and pineal gland (3). The occurrence of intrarenal YST away from the midline is extremely rare, and only three cases of intrarenal YST have been reported (3, 4, 5). The origin of extragonadal YST is not well-understood. It is possible that these tumors arise from germ cells misplaced or arrested in their embryonic migration (2, 3), and the remnant of tissue anywhere along the migration course can become a future site of germ cell tumor.
The reported cases of intrarenal YST only focused on the descriptions of histopathologic findings. One case of a mixed germ cell tumor with calcification and necrosis on CT and sonography has been described (5). No other imaging reports of the intrarenal YST were reported. In the present case, no intratumoral calcification was found. YST was presented as a heterogeneous, enhancing mass with small, patchy or round, lower density areas, corresponding to hemorrhage or necrosis. The margin of the mass was poorly defined, and its substance was less attenuating than the adjacent enhanced, uninvolved renal parenchyma. Some well-enhanced, dilated vessels in the ovarian YST, which was previously described by Choi et al. (6), were also seen in our case. The intratumoral multiple nodules showed ring-shaped enhancement. Typically, the mass grows by infiltration, surrounding and encasing (rather than displacing) the collecting system.
Preoperative knowledge of the intravascular tumor thrombosis may affect the surgical procedure. The ultrasound with color Doppler has been considered as the best technique to detect intracaval tumor thrombus in children with a renal tumor (7). A recent study (8) showed that the contrast-enhanced CT has high sensitivity and specificity for detection of cavoatrial tumor thrombus in Wilms tumor cases. However, in difficult cases where the IVC or renal vein cannot be visualized clearly due to their distortion by large tumors, both Doppler and CT would miss the lesions (7, 8). In our case, both CT and ultrasound identified tumor thrombus of IVC before chemotherapy, but the lesion of the left renal vein was missed due to non-visualization of the left renal vein secondary to mass effect from the tumor.
The main differential diagnosis of intrarenal YST with IVC and left vein thrombus in children is Wilms tumor. Wilms tumor is the most common renal tumors in the pediatric group, accounting for 88% of the cases (9). The two reported cases with intrarenal YST, as well as our case, were all misdiagnosed as Wilms tumor preoperatively or before the cytological diagnosis (3, 5). The other previously reported case mentioned above was diagnosed as a malignant tumor preoperatively (4). The size of the tumor, hemorrhage and necrosis, enhancement model, and vascular involvement did not show any specific pattern to help distinguish YST from Wilms tumor. The previous studies (10) showed that the margins of Wilms tumor were often smooth and well-defined. The tumor grows by expansion, forms a pseudocapsule, usually is distorted, and displaces the collecting system; the invasion of the renal pelvis was rather unusual. These imaging findings were different from those obtained in our case. In addition, the intratumoral multiple nodules with enhancing rim were observed in our case, which may be another differential sign. Other two aggressive renal tumors such as rhabdoid tumor and clear cell sarcoma should be included in the possible differential diagnoses of the intrarenal YST. The distinctive features of rhabdoid tumor include a crescent-shaped fluid collection in the periphery of the tumor, linear calcifications outlining the tumor lobules, a second primary brain tumor, and hypercalcemia (11). Clear cell sarcoma is characterized by its aggressive behavior and is associated with a high rate of bone metastasis. It usually demonstrates a sharply demarcated solid intrarenal mass without intravascular extension (10). Obtaining the serum AFP level would be helpful, as many extragonadal YST were associated with a markedly elevated serum level of AFP, whereas Wilms tumor and other renal masses were not (12).
Yolk sac tumor usually does not respond to radiotherapy, but they respond well to the combination chemotherapy (2). The treatment of choice for YST is neoadjuvant chemotherapy for optimal cytoreduction, followed by resection of the residual tumor. The reduction in tumor size makes surgical resection easier and less extensive. As was shown in our case, preoperative chemotherapy was successful in reducing the tumor of the patient. If the final pathology shows viable tumor cells, the adjuvant chemotherapy is to be added. The serum AFP level is a useful marker for diagnosis, assessing the response to treatment and monitoring the recurrence of intrarenal YST in children.
In conclusion, the intrarenal YST is extremely rare. The appearances on the imaging of the intrarenal YST have many similarities to Wilms tumor. However, the signs on imaging such as lack of pseudocapsule, intratumoral nodules with enhancing rim, encasement of collecting system, and well-enhancing vessels are different from Wilms tumor. If a tumor is observed with these imaging characteristics in children, the serum AFP levels should be checked to help in making a presurgical diagnosis.
Figures and Tables
Fig. 1
Intrarenal YST with intravascular involvement in 2-year-old boy.
A. Color Doppler shows large, heterogeneous mass with marked intralesional flow in left kidney. B. Contrast-enhanced CT scan shows necrotic solid mass with multiple enlarged vessels (short arrows). Extension of lesion into IVC is noted (long arrow). C. Contrast-enhanced CT scan shows two tumor nodules (arrows) with enhancing rim within renal parenchyma. D. Excretory-phase CT scan shows tumor growing around calices (arrows), which are narrowed or obliterated. IVC = inferior vena cava, YST = yolk sac tumor. E. Photomicrograph shows papillary structures that consist of columnar tumor cells, surrounding glumeruloid blood vessels (Schiller-Duval body, arrows) (hematoxylin and eosin, × 200). F. Postcontrast CT scan shows marked reduction in size of mass after chemotherapy. Tumor extending into left renal vein is noted (arrow). G. Gross specimen shows variegated cut surface and multiple tumor lobules with central hemorrhage. Tumor is encasing and infiltrating collecting system, which is consistent with CT findings. YST = yolk sac tumor
References
1. Merchant A, Stewart RW. Sacrococcygeal yolk sac tumor presenting as subcutaneous fluid collection initially treated as abscess. South Med J. 2010; 103:1068–1070.
2. Park NH, Ryu SY, Park IA, Kang SB, Lee HP. Primary endodermal sinus tumor of the omentum. Gynecol Oncol. 1999; 72:427–430.
3. Kumar Y, Bhatia A, Kumar V, Vaiphei K. Intrarenal pure yolk sac tumor: an extremely rare entity. Int J Surg Pathol. 2007; 15:204–206.
4. Radhika S, Bakshi A, Rajwanshi A, Nijhawan R, Das A, Kakkar N, et al. Cytopathology of uncommon malignant renal neoplasms in the pediatric age group. Diagn Cytopathol. 2005; 32:281–286.
5. Liu YC, Wang JS, Chen CJ, Sung PK, Tseng HH. Intrarenal mixed germ cell tumor. J Urol. 2000; 164:2020–2021.
6. Choi HJ, Moon MH, Kim SH, Cho JY, Jung DC, Hong SR. Yolk sac tumor of the ovary: CT findings. Abdom Imaging. 2008; 33:736–739.
7. Solwa Y, Sanyika C, Hadley GP, Corr P. Colour Doppler ultrasound assessment of the inferior vena cava in patients with Wilms' tumour. Clin Radiol. 1999; 54:811–814.
8. Khanna G, Rosen N, Anderson JR, Ehrlich PF, Dome JS, Gow KW, et al. Evaluation of diagnostic performance of CT for detection of tumor thrombus in children with Wilms tumor: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2012; 58:551–555.
9. Jenkner A, Camassei FD, Boldrini R, de Sio L, Ravá L, Bosman C, et al. 111 renal neoplasms of childhood: a clinicopathologic study. J Pediatr Surg. 2001; 36:1522–1527.
10. Lowe LH, Isuani BH, Heller RM, Stein SM, Johnson JE, Navarro OM, et al. Pediatric renal masses: Wilms tumor and beyond. Radiographics. 2000; 20:1585–1603.
11. Agrons GA, Kingsman KD, Wagner BJ, Sotelo-Avila C. Rhabdoid tumor of the kidney in children: a comparative study of 21 cases. AJR Am J Roentgenol. 1997; 168:447–451.
12. Davidoff AM, Hebra A, Bunin N, Shochat SJ, Schnaufer L. Endodermal sinus tumor in children. J Pediatr Surg. 1996; 31:1075–1078. discussion 1078-1079.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download