Abstract
Extramedullary hematopoiesis (EMH) represents tumor-like proliferation of hemopoietic tissue which complicates chronic hemoglobinopathy. Intracranial EMH is an extremely rare occurrence. Magnetic resonance imaging (MRI) offers a precise diagnosis. It is essential to distinguish EMH from other extradural central nervous system tumors, because treatment and prognosis are totally different. Herein, we report the imaging findings of beta-thalassemia in a 13-year-old boy complaining of weakness of left side of the body and gait disturbance; CT and MRI revealed an extradural mass in the right temporoparietal region.
Beta-thalassemia is a hemolytic disease characterized by a gene defect in the production of beta globin chain from hemoglobin, which causes a relative excess of alfa-globin chain, ineffective erythropoiesis, and peripheral destruction of red blood cells. Extramedullary hematopoiesis (EMH) is a process in which the body attempts to maintain erythrogenesis by proliferation of hemopoietic tissue outside the bone marrow in response to chronic anemia (1, 2). This is commonly seen in the liver, spleen, kidney, and lymph nodes (1, 2). Occurrence has also been reported in -the skin, adrenal gland, thymus, ovary, and pelvic region (1, 2). An intracranial EMH is an extremely rare occurrence that frequently involves the cranial dura, falx, cerebral parenchyma, optic nerve sheath, and diploic space of the skull (1-3). Magnetic resonance imaging (MRI) remains the modality of choice in investigations. To our knowledge, this is the largest intracranial EMH foci reported to date with diffusion weighted imaging (DWI) features.
A 13-year-old boy with beta-thalassemia and complaining of progressive weakness of the left side of the body which manifested as gait disturbance and has been progressing over the last year without any increase in intracranial pressure symptoms. The boy's medical history includes a splenectomy performed 10 years earlier, and was on intermittent blood transfusions every two months to correct anemia. At the time of admission, his general condition was fair with a body temperature of 36.2℃, pulse rate of 95 beats/min, respiratory rate of 21/min, blood pressure of 110/70 mm Hg, and pale skin and mucous membrane. On central nervous system (CNS) examination, the boy was fully conscious, and well-oriented with a bilateral normal pupillary size and reaction. Power of the left-sided limbs was graded as 3/5 which is classified as slightly hypertonic and hyporeflexia. The right limbs were normal and other systemic examinations were unremarkable. His blood investigations found a high white blood cell count (14.75 g/L), very low red blood cell count (0.62 T/L), significantly low hemoglobin percentage (10 g/L), very low platelet (73 g/L), low albumin to globulin ratio (6.8), moderately high total bilirubin (42.6 µmol/L), and elevated indirect bilirubin (37.80 µmol/L) and serum iron concentration (43.9 µmol/L).
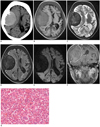
The boy underwent a non-contrast computed tomography (CT) of the head using a Somatom Plus 4 (Siemens Healthcare, Forchheim, Germany). The CT showed a high-attenuating mass with a density of 45 Hounsfield units and a clear margin in the right temporo-parietal region compressing the lateral ventricle and brain parenchyma causing midline shift. The lesion was in close contact with the diploic space of the skull bones, which were thickened and associated with abnormal bony proliferation (Fig. 1A).
MRI was performed using a 3.0-T Signa Excite scanner (GE Medical Systems, Milwaukee, WI, USA). The MRI revealed an extradural mass in the right temporo-parietal region measuring 6.6 × 4.8 cm with a clear boundary and a 5 mm midline shift. In addition diffuse thickening of the skull bones and diploic space was observed. T1-weighted images showed a mass with a slightly high signal intensity compared to gray matter, while low signal intensity was seen on T2-weighted images with no perilesional edema. Fluid attenuation inversion recovery images showed low signal intensity of the mass with adjacent dural high signal intensity. Single shot echo planner DWI obtained at a b-value of 1000 sec/mm2, showed homogeneously low signal intensity which was consistent on an apparent diffusion coefficient (ADC) map. Moderate enhancement of the mass and marked enhancement of the dura was seen on T1-weighted contrast enhanced images (Fig. 1B-F).
A surgical exploration and excision of the mass was performed, and the resected specimen on Hematoxylin and Eosin stain showed multiple foci of mature and immature hematopoietic cells of the erythroid, myeloid and multinucleated megakaryocytic lineages arranged in row patterns (Fig. 1G). About 15-22 megakaryocytes were seen in each high power field. Immunohistochemistry showed scattered positive reaction to CD20, CD38, CD3, CD79a, CD15, CD68 and strong reaction to CD61.
EMH is a process in which the body attempts to maintain erythrogenesis by proliferation of pluripotent stem cells outside the bone marrow due to less production of blood cells in order to meet the body's demand to compensate for anemia. It is observed in hemoglobinopathies (thalassemia, sickle cell anemia, leukemia and hereditary spherocytosis), myeloproliferative disorders (myeloid metaplasia, polycythemia rubravera, myelofibrosis, agnogenic myeloid metaplasia), and in neoplasms involving bone marrow and metastasis (2). There are two forms of EMH; namely the para-osseous in which the normal medullary tissue of the bone ruptures to present as a para-osseous mass, which occurs more frequently in hemoglobinopathies. In addition, extra-osseous masses in which EMH occurs within the soft tissue arising from pluripotent stem cells where marrow activity is ineffective, usually accompany myeloproliferative disorders (2).
Intracranial EMH occurs rarely, and CNS involvement is thought to be the proliferation of pluripotent stem cells, which occurs in the tissue of mesenchymal origin (4). In humans, erythroblasts have been found around the meningeal nerve, thus intracranial EMH in children may be a representation of persistency of the fetal EMH. CNS cells are capable of producing hemopoietic growth factor that may also promote the development of EMH (5). Although patients are clinically silent, signs and symptoms related to raising an intracranial pressure-like headache, visual disturbance, papilledema, motor and sensory impairment, and even coma may develop. Diagnosis of EMH is based on clinical features, laboratory findings, and imaging modalities. The classic peripheral blood smear reveals a leukoerythroblastic picture with immature white and red blood cells, along with circulating megakaryocytes. The bone marrow is often fibrotic and the CT appearance was first described by Lund and Aldridge as multiple extradural masses with an attenuation value equal to or higher than gray matter with marked enhancement after contrast injection (2). MRI remains the modality of choice for showing intermediate to low signal intensity on T1-weighted images and characteristic low signal on T2-weighted images (4). This hypointensity is contributed to by a magnetic susceptibility effect caused by hemosiderin, which is also responsible for low signal intensity in DWI and a drop in ADC (6). Enhancement can be variable on MRI, which may be due to the breakdown of the blood-brain barrier as reported by Koch et al. (). Thickening along the tentorial and falx with moderate enhancement after contrast injection may be seen. The mass may contain adipose tissue but calcification is only a rare finding.
Treatment options for EMH are regular transfusion therapy and low dose radiation therapy (1000-3000 cGy). Hydroxyurea and myelosuppressive agents have successfully been employed for its medical management. Surgical treatment is least recommended due to excessive bleeding and a higher rate of recurrence. EMH usually regresses or disappears after medical treatment.
The differential diagnosis of intracranial EMH includes a meningioma, which mainly affects middle age people, along with a characteristic MRI finding, which is iso to the hypointensity on T1 and hyperintensity on T2, as well as iso to low intensity on DWI, showing strong contrast enhancement. Neuroblastomas, which account for less than 1% of primary CNS tumors and more than 50% manifest in the first 5 years of life. MR imaging shows low signal on T1, T2 weighted images and high signal on DWI with strong contrast enhancement. Some areas may reveal high signal on both T1- and T2-weighted images, suggesting methemoglobin (7). Chloroma is a solid tumor of immature granulocytes and usually occurs in patients with acute myeloid leukemia, which is usually seen in children with a mean age of about 7 to 8 years, typically in the periorbital region. MRI demonstrates hypointensity or isointensity on both T1 and T2-weighed images, and hyperintensity on DWI with homogeneous enhancement following contrast administration (8). Granuloma is isointense to brain parenchyma on T1-weighted MR images and isointense to hypointense on T2-weighted MR images with moderate contrast enhancement. Meningeal metastasis rarely presents as an isolated dural mass and commonly seen in the adult population. It is generally isointense on T1-weighted images, hyperintense on T2 and DWI, showing homogeneous enhancement after contrast injection (9). EMH can be complicated by hemorrhage causing a subdural hematoma (10), and arterial thrombosis or infection. Cases of EMH have also been reported with pilocytic astrocytomas, lipomas, hemangioblastomas (5) and a malignant meningioma (3).
In conclusion, the diagnosis of EMH can be established on the basis of the radiographic view, especially when it occurs in patients with a prolonged history of anemia, or in the region where thalassemia is prevalent. Although MRI remains the modality of choice of investigation, confirmation by biopsy or surgical intervention is mandatory in uncertain cases.
Figures and Tables
Fig. 1
Intracranial EMH in 13-year-old boy with beta-thalassemia.
A. Axial non-contrast CT showing soft tissue mass with density of 45 Hounsfield units in right temporo-parietal region with smooth margin compressing adjacent brain parenchyma. Mass is in close contact with diploic space of skull and displays periosteal reaction. B. T1-weighted MR image showing homogeneously slightly high signal intensity mass compared to gray matter with clear margin in right temporo-parietal region, causing buckling of white matter, ipsilateral ventricle compression, and midline shift. Uniform thickening of diploic space and skull bone is also seen. C-E. Mass showing homogeneously low signal intensity on T2-weighted image (C), fluid attenuation inversion recovery (D) and diffusion weighted imaging (E). F. Contrast enhanced coronal T1-weighted image showing significant dural enhancement and moderate enhancement of mass. G. Pathological examination of postoperative specimen on Hematoxylin and Eosin staining showing round to oval cells with erythroblasts, megakaryocytes and promyelocytes (magnification, × 400).
References
1. Tsitouridis J, Stamos S, Hassapopoulou E, Tsitouridis K, Nikolopoulos P. Extramedullary paraspinal hematopoiesis in thalassemia: CT and MRI evaluation. Eur J Radiol. 1999. 30:33–38.
2. Haidar S, Ortiz-Neira C, Shroff M, Gilday D, Blaser S. Intracranial involvement in extramedullary hematopoiesis: case report and review of the literature. Pediatr Radiol. 2005. 35:630–634.
3. Mathews MS, Duma CM, Brant-Zawadzki M, Hasso A, Westhout FD, Klein DJ, et al. Extramedullary hematopoeisis within a convexity meningioma. Surg Neurol. 2008. 69:522–525. discussion 525.
4. Koch BL, Bisset GS 3rd, Bisset RR, Zimmer MB. Intracranial extramedullary hematopoiesis: MR findings with pathologic correlation. AJR Am J Roentgenol. 1994. 162:1419–1420.
5. Beckner ME, Lee JY, Schochet SS Jr, Chu CT. Intracranial extramedullary hematopoiesis associated with pilocytic astrocytoma: a case report. Acta Neuropathol. 2003. 106:584–587.
6. Kang BK, Na DG, Ryoo JW, Byun HS, Roh HG, Pyeun YS. Diffusion-weighted MR imaging of intracerebral hemorrhage. Korean J Radiol. 2001. 2:183–191.
7. Klisch J, Husstedt H, Hennings S, von Velthoven V, Pagenstecher A, Schumacher M. Supratentorial primitive neuroectodermal tumours: diffusion-weighted MRI. Neuroradiology. 2000. 42:393–398.
8. Smidt MH, de Bruin HG, van't Veer MB, van den Bent MJ. Intracranial granulocytic sarcoma (chloroma) may mimic a subdural hematoma. J Neurol. 2005. 252:498–499.
9. Kremer S, Grand S, Rémy C, Pasquier B, Benabid AL, Bracard S, et al. Contribution of dynamic contrast MR imaging to the differentiation between dural metastasis and meningioma. Neuroradiology. 2004. 46:642–648.
10. Tun K, Kaptanoglu E, Celikmez RC, Gurcan O, Turkoglu OF, Kutluay L. Meningeal extramedullary haematopoiesis mimicking subdural hematoma. J Clin Neurosci. 2008. 15:208–210.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download