Abstract
The radiological appearance of diffuse discrete pulmonary nodules associated with cryptogenic organizing pneumonia (COP) has been rarely described. We describe a case of COP in 49-year-old woman with acute myeloid leukemia who developed diffuse pulmonary nodules during the second course of induction chemotherapy. The clinical status of the patient and imaging findings suggested the presence of a pulmonary metastasis or infectious disease. A video-assisted thoracoscopic lung biopsy resulted in the unexpected diagnosis of COP as an isolated entity. Steroid therapy led to dramatic improvement of the clinical symptoms and the pulmonary lesions.
The typical radiological appearance of cryptogenic organizing pneumonia (COP) is the presence of bilateral peripheral patchy air-space opacities (1). The presence of diffuse nodular infiltration mimicking multiple pulmonary metastases is a rare finding for this disease. We report a case of a patient with acute myeloid leukemia (AML) who presented with this unusual manifestation after chemotherapy to emphasize the importance of early recognition and the use of prompt steroid therapy for this treatable condition.
A 49-year-old, nonsmoking female had been treated for AML with one course of induction chemotherapy with the use of daunorubicin and cytosine arabinoside (ARA-C). After relapse of AML, the patient was treated with 10 mg/m2 IV mitoxantrone daily for six days and 3 g/m2 IV ARA-C, daily for six days. On day six, flu-like symptoms including fever and cough developed after completion of the chemotherapy. On examination, the patient had an elevated temperature (38.5 ℃) and tachypnea. Chest auscultation revealed diffuse bilateral inspiratory crackles. The results of arterial blood gas analysis in room air were as follows: PaO2 92 mmHg, PaCO2 30 mmHg and pH, 7.37. The results of a laboratory evaluation were as follows: hemoglobin level, 104 g/L; platelet count, 140,000/µL; white blood cell count, 11,100/µL. The level of C-reactive protein and the erythrocyte sedimentation rate did not increase. Chest radiograph showed mild linear and small patchy opacities in both lower lung fields. Multiple blood and sputum cultures drawn were all negative. Fiberoptic bronchoscopy showed patent airways and the bronchoalveolar lavage (BAL) fluid was non-diagnostic. Culture and microscopy of sputum and BAL materials were negative for fungi, bacteria, acid-fast bacilli, Pneumocystis carinii and cytomegalovirus. Empirical antibiotic therapy was initiated for a case of presumed pneumonia.
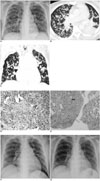
Over the next few days, fever and cough persisted in spite of the antibiotic treatment. On day 11, chest radiograph demonstrated a bilateral and symmetrical distribution of multiple pulmonary nodules (Fig. 1A). Twenty hours later, a chest computed tomography (CT) scan confirmed the presence of numerous discrete 1-mm to 5-mm nodules throughout the lungs (Fig. 1B, C). Due to the poor response to the antibiotic treatment and the questionable diagnosis of pulmonary metastases, a video-assisted thoracoscopic lung biopsy with wedge resection of the posterior segment of the right upper lobe was performed on day 13. Histopathological analysis revealed that polypoid granulation tissue was filling the air spaces and foamy macrophages and inflammatory cells with interstitial pneumonia and air-space fibrosis were present, consistent with COP (Fig. 1D, E). Dramatic improvement of the clinical symptoms and radiographic abnormalities was noted after two weeks of appropriate steroid treatment (Fig. 1F), and the lesions were almost completely resolved after three weeks (Fig. 1G). At a follow-up visit one month after discharge, the patient remained asymptomatic and chest radiograph showed a stable appearance.
Cryptogenic organizing pneumonia is a distinct clinicopathological entity characterized by a subacute illness with shortness of breath, fever, cough, malaise and patchy peripheral air-space infiltrates as depicted on chest radiographs (1). Its full clinical spectrum and course are variable. The disease is defined histopathologically as granulation tissue plugs within the lumina of small airways, alveolar ducts and alveoli (2). Most cases are idiopathic and may be secondary to infections, a hematological malignancy, connective tissue disorders, drug administration, exposure to radiation or bone marrow or lung transplantation (2, 3).
Cryptogenic organizing pneumonia has a variable radiologic appearance and is usually characterized by unilateral or bilateral areas of patchy air-space consolidation with a subpleural and peribronchial distribution (1, 2). In a review of 14 patients with COP, Müller and colleagues (4) found no single finding or combination of findings that could be considered diagnostic of this disease. The presentation of a patient with diffuse discrete 1-mm to 5-mm pulmonary nodules associated with COP is uncommon. Correlation of the high-resolution CT abnormalities with the findings on video-assisted thoracoscopic biopsy suggest that the nodules seen on CT corresponded to localized areas of organizing pneumonia around the bronchiolitis obliterans. This pattern of lesions is separated from other involved bronchioles by a zone of relatively normal parenchyma, explaining the nodular appearance. The findings differ from the patchy or confluent nodular infiltrations usually associated with COP (5, 6). The clinical presentation in this case differs from that of neoplastic diseases, however, due to the rapid progression to diffuse nodules over a few days. Furthermore, multiple nodules are not always a sign of malignant disease, and benign lesions such as tuberculosis and fungal and parasitic infections should be considered in the differential diagnosis. Definitive differentiation of COP from infectious diseases by imaging studies, however, is usually not possible.
The absence of microorganisms in cultures of blood, sputum, and BAL fluid and the failure of empirical antibiotic treatment ruled out active infection at the onset of pulmonary symptoms in our patient. The factors responsible for COP in this case remain unclear but may include a direct immunologic reaction resulting from a possible subclinical viral infection, a toxic reaction to the chemotherapeutic agents and underlying hematological disease. However, whatever the etiology of COP in this case, the clinical symptoms and pulmonary lesions dramatically improved as the patient was responsive to steroid therapy. Further, early diagnosis prevented the patient from having rapidly progressive COP that is a deadly form of the disease and can occur in a small percentage of patients (7).
As pointed out in previous studies, benign pulmonary nodules in patients with an underlying malignancy have occasionally been seen in the setting of allogeneic bone marrow transplantation (8) or in chemotherapeutic drug toxicity (9). In our patient, the question of drug-induced COP arises as bone marrow transplantation was not performed and the clinical and radiological manifestations of pulmonary lesions developed after chemotherapy. Our patient, however, was not given bleomycin, which has been associated with nodular COP mimicking pulmonary metastases (10). The association between the administration of high-dose intravenous ARA-C and lung toxicity is well recognized, and Battistini et al. (11) reported the development of COP in three children with acute leukemia who received a combination of high-dose ARA-C and anthracyclines, which is similar to the regimen that was followed by our patient. Therefore, although undetectable infectious agents may be etiologic factors, chemotherapeutic toxicity can be presumed.
In conclusion, our patient had an unusual radiological pattern of diffuse discrete pulmonary nodules associated with COP that mimicked pulmonary metastases. The development of new pulmonary symptoms accompanied by a radiological manifestation of diffuse pulmonary nodules in cancer patients during chemotherapy should prompt consideration of this diagnosis so that appropriate steroid treatment can be initiated.
Figures and Tables
Fig. 1
Diffuse nodular pattern of cryptogenic organizing pneumonia in leukemic patient in 49-year-old female.
Posteroanterior chest radiograph (A) showing diffuse nodular opacities scattered throughout both lungs. Axial CT (B) and reformatted coronal (C) images of lung show numerous 1-mm to 5-mm nodules (arrow) in diffuse distribution and along bronchovascular structure. Patchy infiltration is also present in left lower lobe. Pathological examination of lung biopsy specimen (D) (Hematoxylin & Eosin staining, ×200) shows aggregates of fibrous plugs within small airways and alveoli around interstitial inflammation, consistent with cryptogenic organizing pneumonia. (E) Parenchymal nodules show localized areas of organizing pneumonia (open arrow) surrounding bronchiolitis obliterans (black arrow) that are separated from other involved areas by zone of relative normal parenchyma (Hematoxylin & Eosin staining, ×40). Two weeks after steroid treatment, chest radiograph (F) shows only few small patchy and nodular opacities in both lungs. Three weeks later, chest radiograph (G) shows almost complete resolution except for linear opacities in right lower lung.
References
1. Preidler KW, Szolar DM, Moelleken S, Tripp R, Schreyer H. Distribution pattern of computed tomography findings in patients with bronchiolitis obliterans organizing pneumonia. Invest Radiol. 1996. 31:251–255.
2. Cordier JF. Cryptogenic organising pneumonia. Eur Respir J. 2006. 28:422–446.
3. Morrish WF, Herman SJ, Weisbrod GL, Chamberlain DW. Bronchiolitis obliterans after lung transplantation: findings at chest radiography and high-resolution CT. Radiology. 1991. 179:487–490.
4. Müller NL, Staples CA, Miller RR. Bronchiolitis obliterans organizing pneumonia: CT features in 14 patients. AJR Am J Roentgenol. 1990. 154:983–987.
5. Akira M, Yamamoto S, Sakatani M. Bronchiolitis obliterans organizing pneumonia manifesting as multiple large nodules or masses. AJR Am J Roentgenol. 1998. 170:291–295.
6. Lee KS, Kullnig P, Hartman TE, Müller NL. Cryptogenic organizing pneumonia: CT findings in 43 patients. AJR Am J Roentgenol. 1994. 162:543–546.
7. Cohen AJ, King TE Jr, Downey GP. Rapidly progressive bronchiolitis obliterans with organizing pneumonia. Am J Respir Crit Care Med. 1994. 149:1670–1675.
8. Tanaka N, Matsumoto T, Miura G, Emoto T, Matsunaga N. HRCT findings of chest complications in patients with leukemia. Eur Radiol. 2002. 12:1512–1522.
9. Epler GR. Drug-induced bronchiolitis obliterans organizing pneumonia. Clin Chest Med. 2004. 25:89–94.
10. Ben Arush MW, Roguin A, Zamir E, el-Hassid R, Pries D, Gaitini D, et al. Bleomycin and cyclophosphamide toxicity simulating metastatic nodules to the lungs in childhood cancer. Pediatr Hematol Oncol. 1997. 14:381–386.
11. Battistini E, Dini G, Savioli C, Morando A, Gandolfo A, Kotitsa Z, et al. Bronchiolitis obliterans organizing pneumonia in three children with acute leukaemias treated with cytosine arabinoside and anthracyclines. Eur Respir J. 1997. 10:1187–1190.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download