Abstract
Objective
This study was designed to evaluate follow-up results in terms of patient prognosis for malignant pulmonary nodules depicted as pure ground-glass opacity (GGO) lesion observed at high-resolution CT (HRCT).
Materials and Methods
Surgical removal for malignant GGO nodules was accomplished in 58 patients (26 men, 32 women; mean age, 57 years; age range, 29-78 years). Patient prognoses were assessed by patient clinical status and the presence of changes in nodule size determined after a follow-up HRCT examination. Differences in patient prognoses were compared for nodule number, size, surgical method, change in size before surgical removal, and histopathological diagnosis by use of Fisher's exact test and Pearson's chi-squared test.
Results
Of the 58 patients, 40 patients (69%) were confirmed to have a bronchioloalveolar carcinoma (BAC) and 18 patients (31%) were confirmed to have an adenocarcinoma with a predominant BAC component. Irrespective of nodule size, number, treatment method, change in size before surgical removal and histopathological diagnosis, neither local recurrence nor a metastasis occurred in any of these patients as determined at a follow-up period of 24 months (range; 12-65 months). Of 14 patients with multiple GGO nodules, all of the nodules were resected without recurrence in six patients. In the remaining eight patients, the remaining nodules showed no change in size in seven cases and a decrease in size in one case as determined after a follow-up CT examination.
Apure ground-glass opacity (GGO) nodule is a round, homogeneous and well-defined hypoattenuating lung lesion with respect to the surrounding soft-tissue structure such as a vessel that has no patches of completely obscured parenchyma (1). Kim et al. (2) have reported that approximately 75% of persistent nodules (with either no change or an increase in diameter for > one month) were either a bronchioloalveolar cell carcinoma (BAC) or an adenocarcinoma with a predominant BAC component (adenoCa with BAC). The remaining 25% of nodules were attributed to atypical adenomatous hyperplasia (AAH) and to a nonspecific fibrosis or an organizing pneumonia (2, 3). Moreover, most of multiple pure GGO nodules (8 of 12, 67%) were BACs or adenoCas with BAC (2).
For the management of BACs or adenoCas with BAC that manifest as pure GGO malignant nodules in the lungs, the appropriateness of limited resection using thoracoscopy for a single malignant GGO nodular lesion has been validated in several studies (4-7). Mun and Kohno (8) have demonstrated that the management of multiple BACs by the use of video-assisted thoracoscopic surgery (VATS) (multifocal wedge resection when the nodules are scattered throughout multiple lobes or both lungs, or a lobectomy when the nodules are localized in a lobe) yielded satisfactory results. However, resective lung surgery should be performed when the patient has reserved lung parenchymal volume. Such treatment may not be feasible when the patient has many nodules, e.g., ten or more nodules. Under these circumstances, chemotherapy may be a valid treatment option (9). Therefore, there is ongoing debate concerning the management of patients with pure GGO malignant nodule(s), particularly when the patients have multiple pure GGO malignant nodules. The purpose of this study was to evaluate the follow-up results in terms of patient prognosis for pure GGO malignant pulmonary nodules that are observed with the use of high-resolution CT (HRCT).
Our institutional review board approved this retrospective study with the waiver of patient informed consent, but written informed consent was acquired from all patients for the CT study.
We reviewed all surgical biopsy files recorded from November 1994 to March 2007 and selected patients who satisfied the following inclusion criteria. 1) Patients were included with pulmonary lesions and the descriptive term "pure GGO nodule(s)" but without any solid or part-solid nodule as noted on the CT reports. 2) Patients with tissue biopsy results of a BAC or adenoCa with BAC were included. 3) Patients who had undergone follow-up CT studies for at least one year after a histopathological diagnosis or management of GGO nodules were included. A GGO nodule (a solid portion < 5% of the nodule volume) was defined as a discrete pulmonary nodular abnormality when it was 3 cm or less in diameter seen with homogeneous attenuation that was not as high as the surrounding soft-tissue structures. A GGO nodule had short- and long-axis diameters within a factor of 1.5 of each other as measured on transverse scans and the GGO nodule was observed on a successive number of thin-section CT scans, thereby verifying that the lesion was spherical. We also included patients with multiple GGO nodules, one or more of which was confirmed as a BAC or an adenoCa with BAC.
A total of 58 patients (26 men, 32 women; mean age, 57 years; age range, 29-78 years) were included in the study. The 58 patients had 106 pure GGO nodules as determined with the use of HRCT. A single nodule was identified in 44 patients and 14 patients had multiple nodules (five patients had two nodules, three patients had three nodules, two patients had four nodules and four patients had five or more nodules) detected by CT. In the 14 patients with multiple GGO nodules, histological confirmation of malignancy, i.e., the presence of a BAC or adenoCa with BAC was determined for at least two nodules in nine patients. In the remaining five patients, histological confirmation of malignancy was obtained for one of multiple GGO nodules, and the other nodule(s) were presumed to be malignant.
Surgical removal of malignant GGO nodule(s) was accomplished in 58 patients. With regard to the 44 patients with a single GGO nodule, 32 patients underwent a lobectomy and 12 patients had wedge resections. Of the 14 patients with multiple GGO nodules, six patients underwent a lobectomy as all of the nodules were localized deep in one lobe or predominant nodule(s) of > 10 mm in diameter were localized in one lobe. Eight patients (including three patients who received multiple-site resections) were treated by wedge resection as the nodules of even size were scattered in multiple lobes or peripherally in one lobe. Of these eight patients, one patient with 15 GGO nodules had undergone chemotherapy with 150 mg/m2 erlotinib (Tarceva; OSI Pharmaceuticals, Melville, NY) daily for 30 months after a VATS wedge resection for one malignant GGO nodule with a peripheral location.
CT scans were obtained with the helical technique using scanners from General Electric Medical Systems (Milwaukee, WI). Units included a single-detector scanner (HiSpeed Advantage; three patients with eight nodules), a four-detector scanner (LightSpeed QX/I; 26 patients with 57 nodules), an eight-detector scanner (LightSpeed Ultra; 13 patients with 16 nodules), a 16-detector scanner (LightSpeed16; 13 patients with 22 nodules) or a 64-detector scanner (LightSpeed VCT; three patients with three nodules). Unenhanced scans only were obtained from two patients with four nodules, and both enhanced and unenhanced scans were obtained from 56 patients with 102 nodules. For an enhancement study, 100 mL of contrast medium (Iomeron 300; Bracco, Milan, Italy) was administered intravenously. For all patients, the scanning parameters were 120 kVp and 70-200 mA. For a single helical CT, a section thickness of 7 mm, a pitch of 1 and a reconstruction interval of 7 mm were used for scanning and image reconstruction. For 4-64 slice CT, a beam width of 10-40 mm, a beam pitch of 1.3-1.5 and a reconstruction interval of 2.5-5.0 mm were used. For single helical CT, additional HRCT scans (1.0-2.5 mm section thickness) were obtained for all of the GGO nodules examined whereas HRCT scans (1.25-2.5 mm) were acquired by retrospective reconstruction of raw data for the 4-64 slice CT imaging. All image data were reconstructed using a bone algorithm.
Two chest radiologists (with two and 18 years experience, respectively) assessed the CT scans retrospectively. Decisions on the CT findings were reached by consensus. CT scans were assessed in terms of size (the longest diameter), location (six lobes, including the lingular division of the left upper lobe as an isolated lobe) and the number of the GGO nodules. The presence or absence of hilar and mediastinal nodal enlargement was evaluated.
CT scans were usually obtained at three- or six-month intervals. Serial CT scans, were available before surgical resection for all patients (follow-up range, 3-72 months; mean, 11 months; median, six months). The presence or absence of size change (the longest diameter when a nodule was single or the longest diameters of the two largest nodules when there were two or more nodules) for the GGO nodules before surgical removal could be evaluated. In addition, any change in attenuation of the GGO nodules was evaluated visually.
Serial CT scans were also available after surgical removal at three or six-month intervals for all patients (follow-up range, 12-65 months; mean, 24 months; median, 21 months). We investigated the presence or absence of local recurrence or a distant metastasis and interval changes in size for the remaining GGO nodules (when none of the nodules could be removed for technical reasons). Patient fates were assessed during the follow-up period.
Entire sections of resected tissues were fixed in formalin and were embedded in paraffin. Several 4-µm-thick sections taken from the equator of the lesions were stained with hematoxylin and eosin and were examined by light microscopy. An experienced lung pathologist (with 15 years of lung pathology experience) interpreted all of the tissue sections. The histopathological diagnoses were classified as BAC, adenoCa with BAC and AAH according to the revised World Health Organization histological classification system (10).
Ten patients had a previous malignant tumor history. Two patients had an adenocarcinoma of the lung, two patients had a papillary carcinoma of the thyroid, three patients had an adenocarcinoma of the colon, one patient had a gastric cancer, one patient had a ductal adenocarcinoma of the distal common bile duct and one patient had an infiltrating ductal carcinoma of the breast. For these ten patients, histological differences between BAC or adenoCa with BAC and previous cancer cell types were clearly shown after a histopathological examination or, if necessary, by immunohistochemical staining for thyroid transcription factor-1 (TTF-1), CK20 or CK7 (11, 12).
Demographic data in terms of the largest nodule size (nodules of < 10 mm and > 10 mm in diameter), surgical method, presence of a size change before surgical removal, pathological diagnosis and gender of patients were compared between patients with single or multiple malignant ground-glass opacity nodules by use of Fisher's exact test and Pearson's chi-squared analysis.
Patient prognoses were assessed by analyzing the presence or absence of local recurrence or a distant metastasis (by assessing patient symptoms and signs, follow-up CT findings and laboratory data), and changes in the size of the remaining GGO nodules (for patients with multiple malignant GGO nodules where all nodules could not be removed surgically) after follow-up HRCT imaging. If there was any recurrence or a metastasis, differences in patient prognoses were compared in terms of nodule number (single versus multiple), size, surgical method used, change in size before surgical removal and histopathological diagnosis.
Histological confirmation was performed on 73 nodules from 58 patients. Of the 73 nodules, 47 were BACs (range of nodule diameter, 6-28 mm; mean, 13 mm; median, 12 mm), 21 nodules were adenoCas with BAC (range of nodule diameter, 5-28 mm; mean, 14 mm; median, 12 mm), and five nodules were AAH (range of nodule diameter, 3-5 mm; mean, 4 mm; median, 4 mm). As for the GGO malignant nodules except for AAH, 28 (48%) patients had nodules < 10 mm in diameter and 30 patients (52%) had at least one nodule > 10 mm in diameter. Patient demographic data for single GGO nodules were not significantly different from data for multiple malignant GGO nodules (Table 1).
Ground-glass opacity nodule(s) were identified on a CT scan in two patients who underwent chest CT for respiratory symptoms, in ten patients who underwent a lung cancer screening and incidentally in 46 patients without pulmonary symptoms who underwent a chest CT examination for other reasons.
Thirty-two patients had BACs and 12 patients had adenoCas with BAC. Twenty-two patients had a nodule < 10 mm in diameter and 22 patients had a nodule > 10 mm in diameter (Table 1). Thirty-six patients had tumors that showed no change in size (over a follow-up period of 1-36 months). Eight patients had tumors that showed an increase in size. There was a 2 mm increase in diameter for six patients over a follow-up period of six to 45 months, a 3 mm increase in diameter for one patient over a follow-up period of 72 months and a 5 mm increase in diameter for one patient over a follow-up period of 42 months before surgical removal. The follow-up period range was 3-72 months; mean, nine months; median, six months. The attenuation value within a nodule did not change in any nodule.
Of the 32 patients who underwent a lobectomy for a single malignant GGO nodule, no patient had a nodal metastasis in the hilum or the mediastinum.
Neither local recurrence nor a metastasis occurred in any of these patients during the follow-up period (range, 12-65 months; mean, 25 months; median 21 months).
Table 2 summarizes the diagnostic methods, HRCT findings for the nodules, previous follow-up study results, management and follow-up results for patients with multiple malignant GGO nodules. Eight patients had BAC(s) and six patients had at least one adenoCa with BAC. Six patients had nodules < 10 mm in diameter and eight patients had at least one nodule > 10 mm in diameter. Ten patients (71%) had tumors that showed no change in size (over a follow-up period of 1-6 months). Four patients (29%) had tumors where at least one nodule had an increase in size. There was a 2 mm increase for two patients over a follow-up period of 27-66 months and a 3 mm increase for two patients over a follow-up period of 18-36 months before surgical removal. The follow-up period range was 3-66 months; mean, 15 months; median, six months (Figs. 1, 2). The attenuation value within a nodule did not change in any nodule.
Of six patients who underwent a lobectomy, none of the patients had a hilar or mediastinal nodal metastasis. All of the multiple nodules were resected in the six patients without recurrence. In the remaining eight patients, the remaining GGO nodules showed no change in size for seven patients, and showed a decrease in size (the largest nodule; from 32 to 20 mm in diameter over a follow-up period of 24 months) in one patient (who received chemotherapy) after follow-up CT imaging (Fig. 3). Neither local recurrence nor a metastasis was detected in any of these patients after a follow-up evaluation (range, 12-41 months; mean, 20 months; median, 18 months).
Neither local recurrence nor a metastasis occurred in any patient with pure GGO malignant nodule(s) as determined after a follow-up evaluation for at least more than one year, irrespective of the nodule number, size, treatment (surgical) method and histopathological diagnosis.
It is currently accepted that limited resection (e.g., a wedge resection or segmentectomy) is the appropriate management strategy for patients with a nonmucinous BAC or an adenoCa with BAC when the lesion appears as a pure GGO nodule (4-7). However, there has been some debate on the management of multiple pure GGO malignant nodules. Multiple limited resections may be feasible if patients have double or triple malignant GGO nodules and pulmonary functional reserve. However, other options should be considered when patients have five or more malignant GGO nodules that are scattered in both lungs with multi-lobar involvement. In our institution, when multiple malignant GGO nodules are localized deep in one lobe or when predominant nodule(s) of > 10 mm in diameter are localized in one lobe, a lobectomy is usually performed. However, when nodules of even size are scattered in multiple lobes or peripherally in one lobe, multiple-site wedge resections are executed.
In one study (9), chemotherapy has been suggested as an alternative therapeutic option. In our study, a patient who had more than 15 malignant GGO nodules in both lungs was treated with chemotherapy (150 mg/m2 Tarceva daily for 30 months). For this particular patient, most of the nodules showed a decrease in size. Furthermore, no additional nodules occurred during the follow-up period of > two years.
In our study, there was no recurrent case after treatment even for patients with multiple GGO nodules (14 patients, 24%). This result concurs with findings of Kodama and colleagues (13). Moreover, in patients who had GGO nodules that remained after surgical resection of one or some of GGO nodules (because all of the nodules could not be resected due to bilateral lung or multi-lobar involvement of the nodules), the remaining nodules did not show a change in size as depicted on follow-up CT images. Therefore, even for multiple malignant GGO nodules, limited resection or follow-up without specific treatment after surgical diagnosis of the lesions may be sufficient as an appropriate management method. However, our results should be interpreted with caution as the follow-up period was short and the number of included cases was small.
Given the excellent prognosis (without mediastinal nodal metastases or without recurrence) of single or multiple malignant GGO nodules, limited resection or imaging follow-up studies without specific treatment after the diagnosis for these nodules may be sufficient. Currently, no optimum follow-up intervals for persistent pure GGO nodules have been reported. As pure GGO malignant nodules have a longer volume doubling time as compared with malignant solid or part-solid nodules (14, 15), follow-up studies with intervals of 12 months should be validated. In our institution, a single pure GGO nodule of 10 mm or less is repeatedly evaluated using HRCT at 12-month intervals.
However, studies have reported that the appearance of a solid portion within a pure GGO area indicates a malignant tumor with a high potential of a loco-regional lymph node or distant organ metastasis (16, 17). Thus, with an option of simple follow-up for these malignant GGO nodules by the use of CT studies particularly when the nodules are > 10 mm in diameter, short-term (e.g., at 3- or 6-month intervals) follow-up evaluation with the use of CT imaging seems more appropriate than a long-term (e.g., at 12-month intervals) follow-up study.
Mun and Kohno (8) experienced newly developed GGO nodules in seven (26%) of 27 patients with multiple BACs after surgical removal of previously present GGO nodules (median follow-up period, 46 months; range 3-81 months). These new lesions were seen as pure GGO nodules of 3 mm or less in diameter (BACs or AAH). These lesions were not invasive in any of the cases, and the patients are still being followed up by the use of CT imaging. In our patients, recurrent GGO nodules were not observed in any patient. This difference may be ascribed to the relatively short follow-up period (range, 12-41 months; mean, 20 months; median, 18 months) in our study.
This study has several limitations. First, the study was performed as a retrospective review. A randomized controlled trial study is needed, particularly for patients with multiple pure GGO malignant nodules. Second, although we included all 14 patients with multiple GGO nodules, this number is still small. In addition, cases were not managed homogeneously. Most patients received surgical resection, while one patient with 15 nodules or more received chemotherapy after a wedge resection.
In conclusion, according to the results of our study, prognoses in patients with malignant pure pulmonary GGO nodules are excellent, and neither local recurrence nor metastases occurs irrespective of nodule number, size, surgical method, presence of size change before surgical removal and histopathological diagnosis. Even for multiple pure GGO malignant nodules or for pure GGO malignant nodule(s) > 10 mm in diameter, minimally invasive surgery with preservation of lung volume and adequate imaging follow-up studies are recommended for both diagnosis and treatment. Another treatment option may be to perform follow-up CT studies only until a solid portion appears within the pure area of ground-glass opacity in order to determine when the surgical removal of the nodules is necessary. However, our results and suggested treatment options for the management of pure GGO malignant nodules are the products of a still evolving study. Long-term follow-up and ideally randomized controlled trial study results remain to be determined.
Figures and Tables
Fig. 1
Multiple (n = 2) ground-glass opacity nodules in right upper lobe; one nodule was identified as adenocarcinoma with predominant bronchioloalveolar carcinoma component (arrow) and other nodule was identified as bronchioloalveolar cell carcinoma (arrowhead). Patient was 59-year-old man (patient number 11 in Table 2).
A, B. Transverse lung window CT (1.25-mm section thickness) scans obtained 15 months before lobectomy for ground-glass opacity nodules show two ground-glass opacity nodules (arrow and arrowhead, respectively) in right upper lobe.
C, D. CT (2.5-mm section thickness) scans obtained at similar levels to A and B and at time of surgery demonstrate that nodules have increased in size. Follow-up CT scans (data not shown) obtained 21 months after right upper lobectomy showed no evidence of local recurrence or distant metastasis.
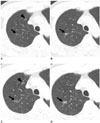
Fig. 2
Multiple (total 8) ground-glass opacity nodules in right upper lobe; two nodules were identified as adenocarcinomas with predominant bronchioloalveolar carcinoma component, five nodules were identified as bronchioloalveolar carcinomas and one nodule was identified as atypical adenomatous hyperplasia. Patient was 49-year-old woman (patient number 13 in Table 2).
A-C. Transverse lung window CT (2.5-mm section thickness) scans obtained through right upper lobe show pure ground-glass opacity nodules (arrows), at least four in number in right upper lobe.
D-F. CT scans (1.25-mm section thickness) obtained at similar levels to and 27 months after A-C demonstrate that most of ground-glass opacity nodules (arrows) have increased in diameter. Follow-up CT scans (data not shown) obtained 18 months after right upper lobectomy disclosed no evidence of local recurrence or distant metastasis.
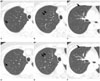
Fig. 3
Multiple (total 15 GGO nodules) malignant ground-glass opacity nodules in 56-year-old woman (patient number 2 in Table 2) who underwent wedge resection for one of nodules in right lower lobe and who received additional chemotherapy.
A-C. Transverse lung window CT (5.0-mm section thickness) scans obtained at levels of thoracic inlet (A), azygos arch (B) and inferior pulmonary vein (C), respectively, show multiple pure ground-glass opacity nodules (arrows) of variable size in both lungs. Wedge resection from one of small ground-glass opacity nodules identified presence of bronchioloalveolar cell carcinoma. Subsequently, patient received chemotherapy.
D-F. Follow-up CT (1.25-mm section thickness) scans obtained 24 months after A-C (with chemotherapy) demonstrate that most nodules (arrows) have decreased in size. In addition, no new nodules appeared during follow-up period. Curved arrow indicates previous wedge resection site.
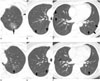
Table 1
Comparison of Demographic Data between Patients with Single or Multiple Pure Ground-Glass Opacity Malignant Nodule(s)

References
1. Henschke CI, Yankelevitz DF, Mirtcheva R, McGuinness G, McCauley D, Miettinen OS. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol. 2002. 178:1053–1057.
2. Kim HY, Shim YM, Lee KS, Han J, Yi CA, Kim YK. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology. 2007. 245:267–275.
3. Lee HJ, Goo JM, Lee CH, Yoo CG, Kim YT, Im JG. Nodular ground-glass opacities on thin-section CT: size change during follow-up and pathological results. Korean J Radiol. 2007. 8:22–31.
4. Asamura H, Suzuki K, Watanabe S, Matsuno Y, Maeshima A, Tsuchiya R. A clinicopathological study of resected subcentimeter lung cancers: a favorable prognosis for ground glass opacity lesions. Ann Thorac Surg. 2003. 76:1016–1022.
5. Ohtsuka T, Watanabe K, Kaji M, Naruke T, Suemasu K. A clinicopathological study of resected pulmonary nodules with focal pure ground-glass opacity. Eur J Cardiothorac Surg. 2006. 30:160–163.
6. Watanabe S, Watanabe T, Arai K, Kasai T, Haratake J, Urayama H. Results of wedge resection for focal bronchioloalveolar carcinoma showing pure ground-glass attenuation on computed tomography. Ann Thorac Surg. 2002. 73:1071–1075.
7. Yoshida J, Nagai K, Yokose T, Nishimura M, Kakinuma R, Ohmatsu H, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg. 2005. 129:991–996.
8. Mun M, Kohno T. Efficacy of thoracoscopic resection for multifocal bronchioloalveolar carcinoma showing pure ground-glass opacities of 20 mm or less in diameter. J Thorac Cardiovasc Surg. 2007. 134:877–882.
9. Gandara DR, West H, Chansky K, Davies AM, Lau DHM, Crowley J, et al. Bronchioloalveolar carcinoma: a model for investigating the biology of epidermal growth factor receptor inhibition. Clin Cancer Res. 2004. 10:S4205–S4209.
10. Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005. 40:90–97.
11. Penman D, Downie I, Roberts F. Positive immunostaining for thyroid transcription factor-1 in primary and metastatic colonic adenocarcinoma: a note of caution. J Clin Pathol. 2006. 59:663–664.
12. Chhieng DC, Cangiarella JF, Zakowski MF, Goswami S, Cohen JM, Yee HT. Use of thyroid transcription factor 1, PE-10, and cytokeratins 7 and 20 in discriminating between primary lung carcinomas and metastatic lesions in fine-needle aspiration biopsy specimens. Cancer. 2001. 93:330–336.
13. Kodama K, Higashiyama M, Yokouchi H, Takami K, Kuriyama K, Kusunoki Y, et al. Natural history of pure ground-glass opacity after long-term follow-up of more than 2 years. Ann Thorac Surg. 2002. 73:386–392.
14. Lindell RM, Hartman TE, Swensen SJ, Jett JR, Midthun DE, Tazelaar HD, et al. Five-year lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers. Radiology. 2007. 242:555–562.
15. Hasegawa M, Sone S, Takashima S, Li F, Yang ZG, Maruyama Y, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol. 2000. 73:1252–1259.
16. Suzuki K, Kusumoto M, Watanabe S, Tsuchiva R, Asamura H. Radiologic classification of small adenocarcinoma of the lung: radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg. 2006. 81:413–419.
17. Kakinuma R, Ohmatsu H, Kaneko M, Kusumoto M, Yoshida J, Nagai K, et al. Progression of focal pure ground-glass opacity detected by low-dose helical computed tomography screening for lung cancer. J Comput Assist Tomogr. 2004. 28:17–23.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download