Abstract
Atherosclerotic coronary artery disease (CAD) is a leading cause of morbidity and mortality globally. Because of its remarkable technological advances, a coronary computed tomography angiography (CTA) is now a crucial imaging tool in the evaluation of patients with nonspecific acute and stable chest pain. This review article provides an overview of atherosclerotic CAD, the pros and cons of CTAs as compared to competing imaging modalities, and the potential remedies to overcome drawbacks of CTAs.
Atherosclerotic coronary artery disease (CAD) comprises subclinical CAD, stable angina and acute coronary syndrome (ACS). ACS is further classified as a ST-elevated myocardial infarction, non-ST-elevated myocardial infarction, and unstable angina (UA). Atherosclerotic CAD is a leading cause of morbidity and mortality in developed countries. Due to recent rapid technological advances, a computed tomography angiography (CTA) enables the evaluation of patients with nonspecific acute and stable chest pain. This article provides an overview of atherosclerotic CAD, the advantages and disadvantages of CTAs as compared to competing imaging modalities, and adjunct methods to overcome the drawbacks of CTAs.
A coronary calcium scoring computed tomography (CT) is a widely accepted imaging tool to diagnose subclinical CAD. Among the various methods, the Agatston score is the most commonly used measure for determining the coronary artery calcium score. Typical CT parameters to acquire the Agatston score are 120 kV with 3 mm collimation and prospective gating without overlapping (1). The Agatston score uses a weighted value assigned to the highest density of calcification [i.e., 1 for 130–199 Hounsfield unit (HU); 2 for 200–299 HU; 3 for 300–399 HU; 4 for 400 HU or greater]. This weighted value is multiplied by the area of coronary calcium (mm2) (1). For instance, if there is a small focus of coronary calcification with an area of 6 mm2 and a peak density of 330 HU in the proximal portion of the left anterior descending artery, the total Agatston score is 18 (6 mm2 × 3). A positive calcium score indicates the presence of asymptomatic subclinical CAD. The calcium score is a surrogate of the total coronary atherosclerotic burden. Thus, a higher Agatston score often indicates a greater risk for future myocardial infarction or cardiac death (2). Radiologists should remember several representative numbers related to the Agatston score. Agatston scores of 100 and 400 indicate moderate and extensive coronary atherosclerotic burden, respectively. Approximately 80–90% of patients with an Agatston score of greater than 400 have at least one significant coronary artery stenosis (50% or more) as indicated by an invasive coronary angiography. In contrast, a negative calcium score indicates a very low likelihood of myocardial infarction and cardiac death in the near future (3). However, one should remember that a 50% or more coronary artery stenosis is not completely excluded in symptomatic patients with a zero calcium score (4).
The Framingham risk score estimates the 10-year risk for future cardiac events by using multiple clinical parameters such as age, sex, serum cholesterol level, smoking history, and blood pressure (5). Aspirin and statins are indicated in asymptomatic subjects with a high Framingham risk score (greater than 20%); whereas, a risk factor modification alone is indicated in asymptomatic subjects with a low Framingham risk score (less than 10%). In the United States, asymptomatic subjects with an intermediate Framingham risk score (10–20%) comprise approximately 40% of adults. In this group, there is no definitive guideline for management based on the Framingham risk score (i.e., risk factor modification versus statin administration). The potential exists for large expenditures if statins are prescribed to all asymptomatic subjects with an intermediate Framingham risk score. Based on the fact that a higher Agatston score indicates a greater likelihood of future cardiac events, the 2010 American College of Cardiology Foundation (ACCF) guideline has classified coronary calcium scoring CTs as appropriate in asymptomatic subjects with an intermediate Framingham risk score (6). The following example explains the incremental value of coronary calcium scoring CT in subjects with an intermediate Framingham risk score. If the Agatston score is zero in an asymptomatic subject with an intermediate Framingham risk score, the subject can be reclassified as at low risk for a future cardiac event. In contrast, subjects with an Agatston score of 1000 and an intermediate Framingham risk score has a high risk for a future cardiac event and a reasonable indication for statin therapy. Radiologists should report an age-sex-matched percentile (7) as well as an absolute Agatston score in their radiological report. A higher agesex-matched percentile (i.e., greater than the 75th percentile) indicates a relatively high risk for future cardiac events. In some countries including Korea, a screening coronary CTA has been widely used (8). However, both the referring physicians and radiologists should bear in mind that there is currently no definite guidelines to recommend its use regardless of the Framingham risk score (9). This is mainly due to the potential hazard of radiation exposure related to cancer risk and its unknown cost effectiveness (8).
The typical plaque that leads to stable angina is fibro-calcific in character (10). Due to the chronic nature of plaque progression, chest pains in patients with stable angina occurs only when myocardial demand exceeds myocardial blood flow (e.g., during emotional or physical stress). Radiologists should understand the concept of typical, atypical, and non-anginal pain in patients with stable angina because the pretest probability of having stable angina is determined by the presence of three factors (i.e., chest pain nature, age, and sex) (11). The need for a coronary CTA in patients with a suspicion of stable angina is determined by the pretest probability. Typical chest pain for stable angina is defined as chest pain having the three following components: typical chest pain quality and aggravating and relieving factors. The typical chest pain for stable angina consists of a substernal squeezing chest pain that lasts approximately 1–5 minutes. An aggravating factor refers to the presence of emotional or physical stress at the time of the chest pains; a relieving factor is considered to be present when the chest pain improves during rest or after administration of a sublingual nitroglycerin. In contrast, atypical and non-anginal chest pain is considered to be present when there are two or fewer of the three factors. It is important to note that a difference in the pretest probability depends upon the sex of the patient, even for patients of the same age and type of chest pain. For instance, the pretest probability for stable angina in a 45-year-old female with typical chest pain is intermediate, whereas it is high in a male. This difference is mainly due to the estrogen protective effect in premenopausal women. Radiologists should keep in mind that a coronary CTA is indicated only in patients with a low to intermediate pretest probability according to the 2010 ACCF guidelines (6). Invasive angiography is often indicated in patients with non-acute chest pain and a high pretest probability, regardless of the result of the treadmill electrocardiogram (ECG) because stress ECG has only moderate accuracy.
Typical chest pain in patients with ACS is similar in nature to that of stable angina. However, compared to the chest pain in patients with stable angina, the chest pain in patients with ACS has a tendency to be more intense and of longer duration, often more than 30 minutes (12). In addition, no definite aggravating or relieving factors exist because ACS frequently occurs after a sudden rupture of a vulnerable plaque in a vessel with mild stenosis (i.e., less than 50% stenosis), which results in extensive coronary artery thrombosis. Radiologists should recognize that the determination of the pretest probability of ACS is different from that for stable angina. A high probability for ACS is defined as a typical substernal squeezing pain lasting more than 30 minutes along with evidence of ischemic ECG changes (e.g., ST depression or elevation, or T wave inversion) or a history of known CAD (e.g., 50% or more coronary artery stenosis on a previous invasive angiography, or history of coronary artery bypass graft or percutaneous coronary intervention). The intermediate pretest probability of ACS is defined as a typical chest pain for ACS lasting more than 30 minutes without ischemic ECG changes or a history of CAD. In contrast, a low pretest probability is defined as atypical chest pain (i.e., typical chest pain lasting less than 30 minutes or atypical chest pain lasting more than 30 minutes) and a lack of evidence of known CAD or ischemic ECG changes (12). In summary, a coronary CTA should be performed only in patients at low to intermediate risk for either stable angina or ACS, but not performed in patients at high risk.
Previous studies have confirmed that a coronary CTA has a negative predictive value of nearly 100 percent (13). This implies that the absence of coronary stenosis on a coronary CTA with excellent image quality obviates further diagnostic evaluation in patients with low to intermediate risk for either stable angina or ACS. Although a coronary CTA has a very high negative predictive value for excluding 50% or more coronary stenosis, there are two possible scenarios where even experienced radiologists may miss significant coronary stenosis. The first scenario is when a motion artifact obscures a significant coronary stenosis and radiologists fail to recognize its presence. The second scenario is if there is 50% or more coronary stenosis over a very short segment. In this case, the stenotic portion may only be identified on a few consecutive axial images with a sub-millimeter collimation (Fig. 1). Therefore, radiologists should undertake a careful review of the axial images so as not to overlook a short segment stenosis. The main purpose of a coronary CTA from the standpoint of the ordering physician is to determine whether invasive angiography is necessary. Thus, radiologists should provide an answer to this question in their CT reports (14). If there is a coronary stenosis of less than 40%, invasive angiography is not indicated. Whereas, it is often indicated in patients with more than 70% stenosis, because the CTA itself does not provide functional information. In patients with intermediate stenosis (40–70%), a functional imaging test such as cardiac single-photon emission computed tomography (SPECT) is often appropriate. An additional important question from ordering physicians is the number of vessels with critical coronary stenosis (more than 70% stenosis). If a patient has a three-vessel disease, a coronary artery bypass graft should be considered (14). Other questions include the major role for a coronary CTA in the emergency department (ED). Can it provide a rapid and safe discharge of patients along with early diagnosis of ACS? Considering that a coronary CTA has a very high negative predictive value, the primary role of a coronary CTA in patients with acute chest pain is to relieve overcrowding in the ED and decrease ED costs more so than the early detection of ACS. The latter scenario is relatively uncommon compared with the former. In addition, a coronary CTA has only moderate specificity to identify 50% or more coronary stenosis because of a blooming or motion artifact (i.e., not negligible false positive rate). Thus, a coronary CTA has limitations in detecting ACS (1516). The coronary CT findings of ACS (both acute myocardial infarction and UA) are significant stenosis (more than 70%) with non-calcified or mixed plaque. In patients with acute myocardial infarction, hypoperfusion without myocardial thinning and regional wall motion abnormality in the territory of a suspicious culprit artery can be identified on a coronary CTA (1517). It is important for radiologists to include an evaluation of the wall motion in their reading process, if the coronary CTA is performed with retrospective ECG-gating. A previous study indicates that diagnostic accuracy is improved with a combined assessment of wall motion and a coronary CTA compared with CTA alone (18). There are three situations in which the evaluation of the wall motion may have an incremental effect. The first is when there is a non-diagnostic coronary segment due to a motion or blooming artifact. In this situation, a regional wall motion abnormality that defines a particular vascular territory can be diagnostic for acute myocardial infarction (Fig. 2). The second situation is in a multivessel disease. Because CTA alone lacks functional information, a significant coronary artery stenosis in combination with regional wall motion abnormality may help to define the culprit vessel. Last, the evaluation of wall motion is beneficial to differentiate ACS from stress-induced cardiomyopathy and myocarditis, which can simulate ACS (15). Moreover, abdominal pain can be a presenting symptom of acute myocardial infarction. Thus, radiologists should consider the possibility of acute myocardial infarction as a cause for nonspecific abdominal or chest pain when they interpret an abdomen CT or non-gated chest CT (Fig. 3). The recommendation to “look at the myocardium always when reading abdomen CT or non-gated chest CT” is highly applicable.
CT has the potential to identify vulnerable plaque as a precursor to ACS. The CT features of a vulnerable plaque are large plaque volume, positive remodeling (remodeling index greater than 1.1), low attenuation plaque that is less than 30 HU (lipid core), and the napkin ring sign (14192021). However, the identification of vulnerable plaque on a coronary CTA remains an emerging concept because of the limited spatial resolution of CTA (14). Furthermore, there is a significant overlap between CT attenuation values of lipid and fibrous plaque (14). This overlap is primarily due to the lipid core being relatively small and located just above a coronary lumen that may be vulnerable to partial volume averaging. The term, napkin ring sign, originates from the morphologic similarity of vulnerable plaque to a napkin ring in some cases. The napkin ring sign is defined as an inhomogeneous plaque with a low attenuation core and high attenuation rim (Fig. 4) (22). However, there is no precise description of the specific CT attenuation value which can be altered depending on the degree of luminal contrast enhancement. Thus, the napkin ring sign may be a more objective sign in determining vulnerable plaque compared with low attenuation plaque. The napkin ring sign is believed to be due to the attenuation difference between the lipid core and fibrous plaque (22). A previous study indicates that the napkin ring sign is an independent predictor of future ACS (21).
A CTA detects coronary atherosclerosis, whereas cardiac SPECT identifies ischemia producing stenosis (i.e., flow limiting stenosis). Thus, the significance of a normal study is quite different for the two techniques. A normal scan in the former often indicates no coronary atherosclerosis; whereas, a negative study in the latter means no functionally significant coronary artery stenosis. Thus, radiologists often encounter a situation where multiple less than 50% stenoses are found on the CTA with a negative result on the cardiac SPECT. However, it is fair to ask whether these insignificant coronary stenosis (less than 50% stenosis) are really insignificant in terms of patient prognosis. The answer is certainly no. One recent study indicated that statin use in patients with non-obstructive CAD identified on the coronary CTA improves outcomes compared with non-statin use (23). One should recognize that the use of only a coronary CTA can noninvasively identify non-obstructive CAD which cannot be visualized on functional noninvasive imaging techniques such as a cardiac SPECT. Thus, this is an obvious advantage of a coronary CTA compared with functional imaging modalities.
A false negative finding on a cardiac SPECT can occur in cases of so-called balanced ischemia. Cardiac SPECT identifies relative perfusion differences between the arterial territories. Thus, if there is no normal reference segment (e.g., three vessel disease or left main disease), a false negative result can occur (24). In contrast, a coronary CTA can easily identify severe multi-vessel coronary involvement.
An important advantage of a coronary CTA compared with competing imaging techniques is its capability to establish an alternative diagnosis that explains the patient's chest pain. Radiologists often encounter a situation where the physician's primary concern is ACS, but the true diagnosis is acute aortic syndrome (Fig. 5) or pulmonary embolism, and the reverse may also occur (25262728). In clinical practice, there are two scan protocols to diagnose ACS (i.e., a dedicated coronary CTA versus triple rule-out CTA). The typical field of view of a dedicated coronary CTA is from the tracheal carina to the heart base; whereas, the triple rule out CTA requires a field of view of nearly the entire chest. Because of the extended z-axis coverage, radiation exposure is higher in a triple rule out protocol compared with a dedicated coronary CTA (2526). Notably, because the field of view of a dedicated coronary CTA includes the lower twothirds of the entire chest, most cases of aortic dissection and pulmonary embolism can be identified on a dedicated coronary CTA (28). Thus, a triple rule out approach should not be used in younger female patients because of the relatively higher risk for radiation which can induce cancer. In summary, the advantages of coronary CTA compared with competing imaging techniques are as follows:
1) High sensitivity and negative predictive value (both for stable angina and ACS)
2) Visualization of coronary wall (vulnerable plaque) noninvasively
3) Identification of non-obstructive CAD (prognostic impact)
4) Identification of balanced ischemia
5) Capability to establish an alternative diagnosis (aortic dissection and pulmonary embolism)
A key shortcoming of a coronary CTA is its limited temporal and spatial resolution. The best temporal and spatial resolution of cutting edge CTs is 66 ms and 0.5 mm, respectively. Thus, advances in these areas of CT are required to improve diagnostic accuracy. More importantly, CT has a relatively low specificity in patients who have a high calcium score (29). Specifically, a blooming artifact (Fig. 6) often results in the overestimation of the severity of stenosis compared with an invasive coronary angiography, which results in an increase in the need for downstream testing (i.e., invasive coronary angiography or cardiac SPECT) (29). In addition, neither a conventional coronary CTA nor an invasive coronary angiography provides hemodynamic significance of coronary stenosis. Although, recent advances such as CT perfusion and CT fractional flow reserve (FFR) allow functional information to be acquired (3031).
Radiologists should remember that the correlation between anatomic coronary stenosis and hemodynamic significance is poor. For instance, in coronary lesions with 50–70% stenosis on an invasive angiography, approximately 40% cause ischemia; whereas, in lesions with 71–90% stenosis, 20% do not produce ischemia (32). This outcome suggests that if a percutaneous coronary intervention were to be used in all patients with a critical stenosis (71–90%), a needless stent insertion would occur in one-fifth of the patients.
Revascularization in ACS has been definitively shown to improve patient outcomes. In contrast, the main purpose of revascularization in patients with stable angina is to relieve angina or reduce the need for urgent coronary intervention rather than improve longevity, even in patients with functionally significant stenoses. The Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation trial enrolled patients with more than 70% coronary artery stenosis and evidence of myocardial ischemia on a treadmill ECG or cardiac SPECT. Patients were randomized into those undergoing a percutaneous coronary intervention with bare metal stents and those receiving optimal medical treatment. In this study, there was no difference between the two groups in terms of major adverse cardiac events (33). The fractional flow reserve versus angiography for guidance of percutaneous coronary intervention in patients with multivessel coronary artery disease (FAME) 1 trial showed FFR-guided stenting had better outcomes than anatomically guided stenting, though fewer drug eluding stents were inserted in the FFR-guided stent group. This result indicates that stenting in patients with functionally nonsignficant stenosis is not benefical and even harmful because of the potential risk for procedure-related complications (34). Because the FAME 1 trial did not directly compare the percutaneous coronary intervention group with the optimal medical treatment group, a FAME 2 trial was peformed (35). Once again, in patients with a stable CAD, there was no solid resulting benefit in the group who underwent a percutaneous coronary intervention using drug eluting stents compared with the optimal medical treatment group (35). Why was there not a solid resulting benefit in the group who underwent a percutaneous coronary intervention in the FAME 2 trial? To answer this question, we need to review the circumstances that produce most clinical events. Most ACS episodes are caused by a rupture of vulnerable plaques (i.e., often less than 50% stenosis) rather than disruption of ischemia-causing plaques (flow-limiting stenosis) (36). Thus, a simple stenting of a flow-limiting stenosis may not dramatically improve future outcomes compared with optimal medical treatment.
In summary, a percutaneous coronary intervention should be reserved for patients with functionally significant stenosis (37). Essentially, the purpose of a percutaneous coronary intervention in patients with stable angina is symptomatic relief or reduction of the risk for an urgent percutaneous coronary intervention as opposed to increased longevity. Furthermore, stenting in cases with functionally insignificant lesions is not beneficial. In this context, a comprehensive evaluation of anatomy and function by a CTA/CT perfusion or CT FFR has the potential to reduce unnecessary invasive coronary angiography and coronary intervention.
If there is a patient with intermediate stenosis (50–70%) in the mid left anterior descending artery, then is revascularization indicated? To answer this question, we need to understand the basic concepts of a CT perfusion and CT FFR.
The basic principle of a stress perfusion CT (Fig. 6) is to maximize the perfusion differences between the arterial territories by using an intravenous adenosine (a short acting vasodilator) to detect a functionally significant coronary stenosis. A typical CT perfusion can be obtained with a stress scan first, but the reverse protocol is also possible (30). Because contrast contamination is avoidable, a stress first protocol is recommended for detecting ischemia causing stenosis. However, administering a rest first scan protocol has an advantage for clinical workflow. If using a rest first protocol, a stress CT perfusion can be limited to patients with 50% or more coronary stenosis. The basic principle of CT perfusion is similar to that of a nuclear myocardial perfusion study. Before a stress perfusion CT, adenosine is infused intravenously for about five minutes. The second scan should be performed at least ten minutes after the first scan to minimize contrast effects from the first scan (30). A stress scan can be done using either a static or dynamic perfusion. In a dynamic CT perfusion, repetitive CT datasets are obtained during the first pass of contrast through the myocardium; whereas in static scanning, the perfusion defect is assessed at one point in time. Thus, the former is able to measure myocardial blood flow by analyzing a time attenuation curve, but it is associated with higher radiation exposure. In contrast, while the latter cannot evaluate myocardial blood flow, it requires less radiation exposure.
The definition of FFR is the ratio of maximum myocardial blood flow in the territory with a coronary artery stenosis to maximum myocardial blood flow in the hypothetical situation of absence of stenosis in the same artery. It can be expressed as the ratio of pressure distal to a stenosis to mean aortic pressure because the relationship between pressure and myocardial blood flow is linear during maximal hyperemia (32). For instance, in a patient whose blood pressure before and after a stenosis is 100 and 70 mm Hg, respectively, the FFR is 0.7 (70/100). We can easily imagine that more functionally significant coronary stenoses tend to induce more compromise in the maximal blood flow of the myocardial territory supplied by the coronary stenosis, which results in a decrease in the FFR. In general, a cutoff value of 0.8 indicates a functionally significant coronary stenosis. Traditionally, FFR is measured using an angiography guided pressure wire (32). However, we can obtain a noninvasive CT FFR from standard coronary CTA data by using computational fluid dynamics. Computational fluid dynamics is the science of predicting fluid flow by solving various mathematical equations (31). Currently, we live at a time when the aerodynamic flow passing by a flying airplane and running car can be precisely calculated. If these are possible, why don't we precisely calculate myocardial blood flow by using coronary CTA data and computational fluid dynamics? This approach is the basic concept of CT FFR (Fig. 7). CT FFR can be derived from CT angiographic anatomy and patient specific physiologic information without the need for protocol modifications such as additional contrast, radiation exposure, or administration of adenosine. This method requires a standard coronary CTA and some turn-around time due to the extensive computational requirements (31). Recently, several venders have developed more rapid onsite software capabilities; however, the diagnostic performance of these newer algorithms needs to be tested in clinical trials.
A blooming artifact is a major obstacle of a CTA in the evaluation of coronary stenosis but can be reduced by the application of dual energy CT techniques (38). However, it results in higher radiation exposure compared with the conventional mode, and limitations based on heart rate are still problematic. Only a few multicenter trials for CT perfusion and CT FFR have been published, most of which have been for CT FFR. Thus, further randomized trials are required to validate the diagnostic performance of these techniques before fully introducing them into clinical practice. Several prospective trials are under way for this purpose. In addition, CT techniques to improve temporal and spatial resolution and scan coverage are ongoing. Thus, the future of coronary CTA is quite promising. Coronary CTA was originally envisioned as a gatekeeper for invasive coronary angiography. However, it has not been entirely successful for this purpose due to the overestimation of stenosis severity and lack of functional information. The advent of a combined CTA/CT perfusion or CT FFR will allow us to revisit this goal.
The following strategy may be effective. If a coronary CTA is normal, patient reassurance is all that is required. If a coronary CTA shows nonsignificant stenosis (less than 50%), statin use is recommended. If there is a 50–89% stenosis on the CTA, CT perfusion or CT FFR can be performed to obtain functional information and permit triage. With this strategy, we can reduce unnecessary invasive coronary angiographies and percutaneous coronary interventions, and as a result, reduce healthcare costs and increase appropriate resource utilization.
In conclusion, a coronary CTA is an accurate and cost-effective method to evaluate atypical chest pains in patients at low to intermediate risk. The time may arrive in the near future when a comprehensive CT evaluation of both anatomy and function may substitute for a diagnostic invasive coronary angiography. Invasive coronary angiography may then be reserved primarily for coronary intervention.
Figures and Tables
Fig. 1
60-year-old male patient with short segment critical stenosis (greater than 70%) in the mid-anterior descending coronary artery.
Arrow in (A) on an axial CT image at the level of the left atrium indicates the mild-anterior descending coronary artery. Pinpoint narrowing at the origin of the mid-anterior descending coronary artery (arrow, B) is demonstrated on a consecutive axial CT image next to (A). The diameter of the mid-anterior descending coronary artery (arrow, C) suddenly returns to normal in the consecutive axial CT image adjacent to (B). Such an abrupt diameter change indicates a very short segment coronary stenosis. This lesion was missed on a coronary computed tomography angiography performed one and a half years earlier. Note that the critical stenosis (arrow) is identified on only one (D) of the two invasive coronary angiographic views (D, E) due to the overlapping of the coronary arteries. Thus, radiologists should be careful not to overlook such short segment stenoses by carefully evaluating all axial raw data.
CT = computed tomography
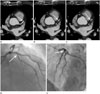
Fig. 2
72-year-old male patient with acute myocardial infarction.
This patient presented to the outpatient department with chest pain that developed four days earlier. The attending physician ordered an elective coronary CTA to rule out stable coronary artery disease. However, the coronary CTA was suboptimal due to a high heart rate (71 bpm). Note the significant motion artifact (arrowheads) along the middle right coronary artery on the curved multi-planar reformatted image (A). There is suspicion for a sub-endocardial perfusion defect (arrowheads, B) in the inferior wall of the left ventricle on the axial CT image with a wide field of view at the level of the left ventricle. However, a beam hardening artifact frequently occurs at this location. Thus, an artifact is not reliably excluded in this patient. On the cine images of the mild-portion of the left ventricle (C, D; diastolic and systolic image, respectively), hypokinesia is noted in the territory of the right coronary artery (i.e., interventricular septum, inferior, and inferolateral wall of the left ventricle). Note there is only mild wall thickening (arrowheads, D) in the corresponding segments compared with the normal systolic wall thickening in the antero-lateral wall of the left ventricle (D). This observation indicates the need to evaluate all phases of the axial raw data. On the short segment curved multi-planar reformatted image with the least motion artifact, findings are suspicious for a critical stenosis (arrows, E) at the distal portion of the middle right coronary artery, even though a motion artifact is present. This result was communicated to the attending cardiologist and emergent invasive coronary angiography confirmed the CT findings (arrows, F). This example shows that an analysis of the regional wall motion abnormality on a coronary CTA enhances the identification of acute coronary syndrome in cases involving a motion or blooming artifact.
CT = computed tomography, CTA = computed tomography angiography
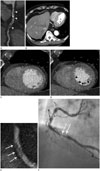
Fig. 3
87-year-old male patient with acute myocardial infarction identified on an abdomen CT. The patient was hospitalized for a fracture of the right femoral neck and developed sudden abdominal pain and shock during hospitalization. The attending physician ordered an abdomen CT. Diffuse subendocardial hypoperfusion (arrowheads) is noted on an axial CT image at the level of the left ventricle, which indicated a myocardial infarction due to the obstruction of the left main coronary artery. At follow-up, an electrocardiogram showed an ischemic pattern and the serum troponin increased. The patient ultimately died due to heart failure.
CT = computed tomography
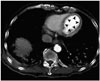
Fig. 4
Napkin ring sign in a 60-year-old male patient on a coronary computed tomography angiography.
A typical napkin ring sign is noted in the distal portion of the mid right coronary artery on the axial CT image at the level of the coronary sinus (arrows, A) and on the curved multiplanar reformatted image (arrowheads, B).
CT = computed tomography
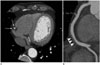
Fig. 5
56-year-old male patient with Stanford type B aortic dissection identified on a dedicated coronary CTA. The primary concern of emergency department physician was acute coronary syndrome. Thus, a dedicated coronary CTA was performed. However, the coronary arteries (not shown) were normal. Instead, a Stanford type B aortic dissection (arrowheads) is identified on a sagittal image using a wide field of view. Note the typical z-axis coverage of a dedicated coronary CTA includes only the lower two-thirds of the entire chest.
CTA = computed tomography angiography
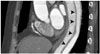
Fig. 6
62-year-old female patient with severe coronary calcifications.
On the curved multi-planar reformatted images, multiple calcified plaques are noted along the left anterior descending (arrows, A), left circumflex (arrows, B), and right coronary artery (arrow, C). Due to a blooming and/or motion artifact, the presence of 50% or more coronary stenosis is not reliably excluded in this patient. A stress CT perfusion has an important role in this occasion. On a short-axis view of a stress CT perfusion (D), there is a perfusion defect only in the territory of the left anterior descending coronary artery (arrowheads). Thus, calcified plaques in the left circumflex and right coronary artery are not hemodynamically significant (case courtesy by professor Sung Mok Kim, Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea).
CT = computed tomography
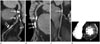
Fig. 7
50-year-old male patient with intermediate stenosis (50–70%) in the middle left anterior descending artery.
Is revascularization appropriate in this patient? On a curved multi-planar reformatted image, a 50–70% stenosis with noncalcified plaque (arrows, A) is noted in the middle left anterior descending coronary artery. The computed tomography FFR value (B) is 0.69 which indicates hemodynamically significant stenosis. Thus, a percutaneous coronary intervention would be recommended in this patient. In fact, the FFR value on a coronary angiography was 0.7 in this patient (case courtesy by professor Dong Hyun Yang, Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea).
FFR = fractional flow reserve
References
1. Lee J. Coronary artery calcium scoring and its impact on the clinical practice in the era of multidetector CT. Int J Cardiovasc Imaging. 2011; 27:Suppl 1. 9–25.
2. Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007; 49:1860–1870.
3. Hoffmann U, Brady TJ, Muller J. Cardiology patient page. Use of new imaging techniques to screen for coronary artery disease. Circulation. 2003; 108:e50–e53.
4. Lee HY, Song IS, Yoo SM, Rho JY, Moon JY, White CS. Can the extent of epicardial adipose tissue thickness or the presence of descending thoracic aortic calcification predict significant coronary artery stenosis in patients with a zero coronary calcium score on multi-detector CT? Atherosclerosis. 2010; 212:495–500.
5. Budoff MJ, Gul KM. Expert review on coronary calcium. Vasc Health Risk Manag. 2008; 4:315–324.
6. American College of Cardiology Foundation Appropriate Use Criteria Task Force. Society of Cardiovascular Computed Tomography. American College of Radiology. American Heart Association. American Society of Echocardiography. American Society of Nuclear Cardiology. North American Society for Cardiovascular Imaging. Society for Cardiovascular Angiography and Interventions. Society for Cardiovascular Magnetic Resonance. Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010; 56:1864–1894.
7. Wong ND, Budoff MJ, Pio J, Detrano RC. Coronary calcium and cardiovascular event risk: evaluation by age- and sex-specific quartiles. Am Heart J. 2002; 143:456–459.
8. Yoo SM, Lee HY, White CS. Screening coronary CT angiography: possibilities and pitfalls. Int J Cardiovasc Imaging. 2014; 30:1599–1601.
9. Korean Society of Radiology. Korean Society of Cardiology. Kim YJ, Yong HS, Kim SM, Kim JA, Yang DH, Hong YJ. Korean guidelines for the appropriate use of cardiac CT. Korean J Radiol. 2015; 16:251–285.
10. Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013; 368:2004–2013.
11. Wake R, Iida H, Ogata H, Takeshita H, Kusuyama T, Kohno H, et al. Cardiac computed tomography for the diagnosis of coronary artery atherosclerosis. Int J Clin Med. 2013; 4:183–189.
12. Chang HJ, Chung N. Clinical perspective of coronary computed tomographic angiography in diagnosis of coronary artery disease. Circ J. 2011; 75:246–252.
13. Hulten EA, Carbonaro S, Petrillo SP, Mitchell JD, Villines TC. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol. 2011; 57:1237–1247.
14. Kim HR, Yoo SM, Rho JY, Lee HY, White CS. MDCT evaluation of atherosclerotic coronary artery disease: what should radiologists know? Int J Cardiovasc Imaging. 2014; 30:Suppl 1. 1–11.
15. Yoo SM, Chun EJ, Lee HY, Min D, White CS. Computed tomography diagnosis of nonspecific acute chest pain in the emergency department: from typical acute coronary syndrome to various unusual mimics. J Thorac Imaging. 2017; 32:26–35.
16. Staniak HL, Bittencourt MS, Pickett C, Cahill M, Kassop D, Slim A, et al. Coronary CT angiography for acute chest pain in the emergency department. J Cardiovasc Comput Tomogr. 2014; 8:359–367.
17. Chandarana H, Srichai MB. Evaluation of myocardial abnormalities and ischemia. Radiol Clin North Am. 2010; 48:771–782.
18. Seneviratne SK, Truong QA, Bamberg F, Rogers IS, Shapiro MD, Schlett CL, et al. Incremental diagnostic value of regional left ventricular function over coronary assessment by cardiac computed tomography for the detection of acute coronary syndrome in patients with acute chest pain: from the ROMICAT trial. Circ Cardiovasc Imaging. 2010; 3:375–383.
19. Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007; 50:319–326.
20. Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009; 54:49–57.
21. Otsuka K, Fukuda S, Tanaka A, Nakanishi K, Taguchi H, Yoshikawa J, et al. Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome. JACC Cardiovasc Imaging. 2013; 6:448–457.
22. Maurovich-Horvat P, Schlett CL, Alkadhi H, Nakano M, Otsuka F, Stolzmann P, et al. The napkin-ring sign indicates advanced atherosclerotic lesions in coronary CT angiography. JACC Cardiovasc Imaging. 2012; 5:1243–1252.
23. Hwang IC, Jeon JY, Kim Y, Kim HM, Yoon YE, Lee SP, et al. Statin therapy is associated with lower all-cause mortality in patients with non-obstructive coronary artery disease. Atherosclerosis. 2015; 239:335–342.
24. Hecht HS. A paradigm shift: coronary computed tomographic angiography before stress testing. Am J Cardiol. 2009; 104:613–618.
25. Lee HY, Yoo SM, White CS. Coronary CT angiography in emergency department patients with acute chest pain: triple rule-out protocol versus dedicated coronary CT angiography. Int J Cardiovasc Imaging. 2009; 25:319–326.
26. Yoo SM, Rho JY, Lee HY, Song IS, Moon JY, White CS. Current concepts in cardiac CT angiography for patients with acute chest pain. Korean Circ J. 2010; 40:543–549.
27. Yoo SM, Lee HY, White CS. MDCT evaluation of acute aortic syndrome. Radiol Clin North Am. 2010; 48:67–83.
28. Lee HY, Song IS, Yoo SM, Rho JY, Moon JY, Kim IJ, et al. Rarity of isolated pulmonary embolism and acute aortic syndrome occurring outside of the field of view of dedicated coronary CT angiography. Acta Radiol. 2011; 52:378–384.
29. Meijs MF, Meijboom WB, Prokop M, Mollet NR, van Mieghem CA, Doevendans PA, et al. Is there a role for CT coronary angiography in patients with symptomatic angina? Effect of coronary calcium score on identification of stenosis. Int J Cardiovasc Imaging. 2009; 25:847–854.
30. Techasith T, Cury RC. Stress myocardial CT perfusion: an update and future perspective. JACC Cardiovasc Imaging. 2011; 4:905–916.
31. Nørgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (analysis of coronary blood flow using CT angiography: next steps). J Am Coll Cardiol. 2014; 63:1145–1155.
32. Pijls NH, Tanaka N, Fearon WF. Functional assessment of coronary stenoses: can we live without it. Eur Heart J. 2013; 34:1335–1344.
33. COURAGE Trial Research Group. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007; 356:1503–1516.
34. FAME Study Investigators. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van't Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009; 360:213–224.
35. FAME 2 Trial Investigators. De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, et al. Fractional flow reserveguided PCI for stable coronary artery disease. N Engl J Med. 2014; 371:1208–1217.
36. King SB 3rd. Is it form or function?: the “COURAGE” to ask. JACC Cardiovasc Interv. 2014; 7:202–220.
37. American College of Cardiology Foundation. American Heart Association Task Force on Practice Guidelines. American College of Physicians. American Association for Thoracic Surgery. Preventive Cardiovascular Nurses Association. Society for Cardiovascular Angiography and Interventions. Society of Thoracic Surgeons. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012; 60:e44–e164.
38. Yunaga H, Ohta Y, Kaetsu Y, Kitao S, Watanabe T, Furuse Y, et al. Diagnostic performance of calcification-suppressed coronary CT angiography using rapid kilovolt-switching dual-energy CT. Eur Radiol. 2017; 27:2794–2801.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download