Abstract
Purpose
The aim of this study was to evaluate the ultrasonographic (US) features of complications associated with injectable fillers in the cervicofacial region.
Materials and Methods
This retrospective study was approved by our Institutional Review Board. Thirty-nine patients (37 females and 2 males; mean age: 52 years; age range: 26–83 years), both symptomatic (n = 32) and asymptomatic (n = 7), who underwent cosmetic procedures in the cervicofacial area and US, were included.
Results
All of the cases were classified into five types based on major US features, including (the most common feature) diffuse high echogenicity with posterior snow storming (n = 17, 39%). The other types included a hypoechoic nodule with high echoic lines (n = 10, 23%), a round hypoechoic nodule (n = 12, 27%), a heterogeneously echogenic lesion with internal echoic dots (n = 1, 2%) and an ill-defined non-mass-like fatty lesion (n = 4, 9%). The time between cosmetic injection and evaluation was variable (range: 3 weeks–21 years). Usually, the injected material was either unknown or illegal (n = 24). Ten patients underwent pathological evaluation. Many histological types of foreign body granulomas were evident.
Cosmetic filler injection is a relatively noninvasive and simple means of facial augmentation; some fillers are synthetic, whereas others are derived from biological materials. These procedures are commonly performed; various filler materials are currently available, and they all have unique qualities, advantages, and disadvantages (123). Once injected, the fillers vary in their lifespan depending upon the material. Fillers are usually injected into the perioral, periocular, and cheek areas of middle-aged women to smooth wrinkles, and the effect of cosmetic augmentation is an enhanced facial appearance. An increasing number of complications has been reported (45). The exact details of procedures and types of agents used are seldom reported or remembered by patients. Asymptomatic lesions are found incidentally during subsequent examinations. It is important for radiologists to be aware of the imaging features of complications associated with commonly used injectable fillers to avoid confusion with true pathologies.
The aim of the present study was to evaluate the ultrasonographic (US) features of complications associated with injectable fillers in the cervicofacial region.
Our Institutional Review Board approved this retrospective study, and the requirement for obtaining informed consent was waived.
The criteria for inclusion were US detection of foreign material within the skin that disrupts the normal tissue plane and confirmation of a previous filler injection by the patient or the referring physician.
Thirty-nine patients were evaluated between July 2008 and September 2014; there were 37 females and 2 males with a mean age of 52 years (range, 26–83 years). The time between filler injection and imaging ranged from 3 weeks to 21 years. Seven patients had no symptoms, and US was performed to examine the other cervicofacial areas. The most common symptoms in the other 32 patients were a palpable mass or mass migration (n = 21, 54%); the other symptoms are summarized in Table 1. One of the four patients who presented with inflammation did not initially disclose her filler injection history. During US, she confirmed that she, in fact, had such a history, and a biopsy was performed. Common sites of injection included the forehead, nasal root, nasolabial folds, cheek, lip, and anterior neck.
When abnormal US features were noticed, a history of cosmetic filler injection was taken during the examinations, and, whenever possible, the specific filler used was recorded. Most of the patients could not remember the specific filler type used (n = 24, 55%), and medical records did not indicate the specific filler type used. Table 2 summarizes the specific fillers recalled by the patients.
US and Doppler examinations were performed using a 5- to 12-MHz linear-array transducer (IU21, 22 US or HDI 5000; Philips Healthcare, Amsterdam, the Netherlands), the Aplio 500 system (Toshiba Medical Systems Corporation, Nasushiobara, Japan), or a 6- to 15-MHz linear array transducer (Logiq E9; GE Healthcare, Little Chalfont, UK). US examinations were performed by a single head-and-neck radiologist with 24 years of experience in order to evaluate the symptoms complained of by patients. Asymptomatic cases were included if US yielded abnormal findings or if a history of cosmetic filler injection was reported.
Histopathological data of 10 incisional or excisional biopsy specimens were reviewed by a single board-certified pathologist unblinded to the clinical or radiological findings. To clarify the causes of the pathological features, including vacuoles and chronic inflammation, immunohistochemistry and Alcian blue staining were performed in five cases to determine whether it would be helpful to distinguish the injected filler materials.
On US, the features of dermal filler injection were classified into the following five types, as summarized in Table 3: diffuse high echogenicity with posterior snow storming (n = 17) (Fig. 1); a hypoechoic nodule with anterior and posterior high echoic lines (n = 10) (Fig. 2); a round hypoechoic nodule (n = 12) (Fig. 3A); a heterogeneously hyperechoic lesion with internal echoic dots (n = 1) (Fig. 4A); and an ill-defined nonmass-like fatty lesion (n = 4). Two of the four patients presenting with a nonmass-like fatty lesion on US (Fig. 5) had histories of autologous fat or lipocyte stem cell transplantation. In types 1 and 2, the normal anatomical layers of the involved face or the cervical region were disrupted due to increased echogenicity of the subcutaneous tissue and marked posterior acoustic shadowing. Palpable masses were located in the skin and the subcutaneous fatty layer.
Histopathologically, chronic inflammation was evident in 10 cases, and it featured fibrosis, the presence of multinucleated giant cells, and vacuoles. Many histological types of foreign body granulomas were evident, depending on the type of filler used. These included classic giant cell granulomas associated with the use of new fillers (Fig. 4B) and cystic and macrophagic reactions associated with the use of liquid silicone (Fig. 6). Another case showed multiple, angulated foreign body materials with foreign body granulomas, multinucleated giant cells, and an asteroid body (Fig. 3B). To enhance the diagnostic accuracy, immunohistochemistry of five cases was performed; all of the results were negative. Neither histology nor immunohistochemistry was useful for identifying the type of the injected filler.
Facial fillers have certain characteristics and imaging features, and US may be used to detect and characterize various filler agents (6789). The biological hyaluronic acid yields anechoic structures on US that are sonographically similar to cysts but lack a thin wall or debris, whereas synthetic fillers generate stronger echoes and (different) predominant posterior artifacts (8). Cosmetic fillers are used predominantly in the skin and the subcutaneous layer, with a broad distribution of filler particles throughout the facial tissues; particle aggregation or scattering may compromise cosmetic success (8).
Although fillers are expected to be biologically inert, skin biopsies consistently show signs of inflammation, either nonspecific in nature or reflecting foreign body granulomatous reactions (8).
Complications associated with filler injection may occur predominantly in patients who are esthetically attentive and aware of the details of the cosmetic or therapeutic procedures. Fortunately, the incidence of complications is low, but the number of reports of such complications appears to be increasing (45781011121314). All facial fillers can cause both early and late complications. Early complications (which develop days to weeks after injection) include immediate hypersensitivity reactions, infections, skin necrosis, overcorrection, and discoloration. Late complications (which develop weeks to years after injection) include infections, filler migration, foreign body granulomas, and scarring (1516). Overfilling (overcorrection) can lead to lumpiness, rendering the filler material conspicuous under the skin surface and triggering patient dissatisfaction. Imaging would be useful to delineate the extent of excess material, identified via contour deformities on the overlying skin. If the degree of inflammation is mild, the condition is usually transient. However, filler agents can elicit more severe chronic inflammatory responses associated with swelling (1718).
Filler migration is cosmetically unattractive, uncomfortable, and debilitating. In the present study, a palpable mass and filler migration were the most common presenting symptoms. Foreign body granulomas are late complications that develop several months to years after injection (1920). The pathogenesis of these reactions remains unknown; several theories exist, including immunological cross-reactions, delayed stimulation of immunity, and cross-contamination via injection (21). Scarring is perhaps caused by chronic inflammation and lymphatic obstruction that develop years after filler injection (101622). In our present study, most of the symptomatic patients who presented with palpable masses or swelling and inflammation exhibited nodular lesions on US and foreign body granulomas with acute or chronic inflammation on pathology examination.
In the present study, various US findings could be interpreted as evidence for the presence of foreign body granulomas with various types of vacuoles, fibrosis, and inflammation, although pathological data were available in only 10 patients. Although various types of foreign body granulomas may exist, histopathological analysis could not identify the specific types of injected cosmetic fillers used.
The imaging features of foreign body granulomas are variable, and include solid-to-cystic round or ovoid foci with irregular microcalcifications and small ring-like, large eggshell calcifications with surrounding fibrosis (23). US provides not only clear visualization of the skin layer and the underlying tissue, but also detection of common filler agents, complications associated therewith, blood flow data, and inflammation (242526). US assists in the monitoring of filler extension when further medical or surgical management is considered (2728). In the present study, US showed that complications associated with filler characteristics vary. If a palpable mass is present, US reveals a hypoor hyper-echoic solid nodular lesion or a low-echoic round nodule associated with the disruption of normal tissue planes. Foreign materials are usually located in the subcutaneous fatty layer and they have a typical distribution. Fatty masses were evident in patients who complained of overcorrection after autologous fat or lipocyte stem cell transplantation. Fatty lesion after lipocyst transplantation presents as unusual thickening of the subcutaneous fatty layer and a nodular lesion with an ill-defined margin unlike lipoma. Therefore, if a fatty lesion is seen during US examination, evaluation of the margin and clinical history taking of fat injection are needed to rule out complication of filler injection.
Traditionally, histology is the most important tool for the evaluation of filler-associated complications in patients in whom a history of a prior cosmetic procedure correlates with clinicopathological findings (29). However, patients who have undergone cosmetic interventions usually tend to avoid invasive procedures such as biopsies, particularly in the highly exposed areas. This may explain why only 10 cases in the present study underwent pathological examination. Even if a biopsy is performed, the information derived therefrom is limited to the area from where the piece of skin was taken (30). In a histopathological and clinical study of orofacial granulomas that develop after cosmetic filler injection, two types of granulomas were evident: the classic foreign body granuloma (CFBG), with numerous giant cells located around the foreign bodies, and the cystic and macrophagic granuloma (CMG), featuring extracellular microcysts surrounded by a (principally) mononuclear infiltrate of vacuolated macrophages (5).
CFBG type 2 is characterized by multiple translucent pinkish particles unevenly distributed on a background of finely fibrillar collagen, with variable lymphocytic infiltrate (5); these findings are similar to those in one of our cases (Fig. 3C). This patient exhibited an asteroid body with multinucleated giant cells and allegedly had a history of collagen injection. The CMG type of liquid silicone granuloma shows a particular pattern of round holes differing in diameter on a background of a foamy infiltrate. This “Swiss cheese pattern” is complicated by extracellular microcysts, with empty cavities rimmed by a thin layer of collagen. The microcysts and vacuoles appear to be empty because the injected silicone oil has been completely drained (Fig. 6) (5). Type 1 or 2 US findings may reflect the use of a mixture of silicone (oil or solid) (810). In most patients belonging to these categories, injected fillers are illegal or unknown. We suggest that liquid silicone is commonly used as an illegal filler.
Our study has some limitations. There could be selection bias because the number of enrolled patients was small. Also, it would be difficult to generalize our result because most of the patients could not identify the type of filler they had received; therefore we could not correlate the US and pathological features with the filler type.
In conclusion, US can demonstrate whether a filler is present and can identify complications associated with filler injections. The knowledge of variable features of complications associated with cosmetic fillers may reduce the possibility of misinterpreting the injected areas as true lesions, in turn avoiding inappropriate medications and reducing unnecessary procedures seeking to rule out true pathological conditions.
Figures and Tables
Fig. 1
A 57-year-old woman underwent US for evaluation of migration of nodules in the forehead and the nasal root. She had a history of illegal injections into the forehead, nose, both nasolabial folds and chin. Transverse US scan of the chin reveals diffuse high echogenicity with posterior snow storming (type 1).

Fig. 2
A 51-year-old woman had a history of illegal filler injection into her cheeks, chin, and forehead 8 years earlier. She complained of migration of the injected materials and a palpable mass in her right cheek. A transverse US scan reveals a hypoechoic nodule with anterior and posterior high echoic lines (type 2, arrows).
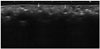
Fig. 3
A 66-year-old woman had a history of collagen injection into the forehead, both glabellae, nasolabial folds, and the upper lip 4 years earlier. She underwent US because of a palpable mass in the forehead.
A. A transverse scan of the forehead reveals a round hypoechoic nodule (type 3).
B. Angulated foreign materials with multinucleated giant cells and an asteroid body are observed (hematoxylin and eosin staining, × 200).
C. The foreign materials are negative for Alcian blue staining (× 200).

Fig. 4
A 49-year-old woman complained of a palpable mass in nasolabial folds of 1–2 months duration. She had been injected with collagen and steroid.
A. A transverse US scan reveals an heterogeneously hyperechoic lesion with internal echoic dots (type 4).
B. Biopsy reveals both acute and chronic inflammation and several multinucleated giant cells (hematoxylin and eosin staining, × 200).

Fig. 5
A 33-year-old woman complained of a palpable mass beneath the lower eyelid. She had a history of autologous fat injection at the same site 9 months earlier. US shows an ill-defined, nonmass-like fatty lesion (type 5, arrows).
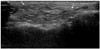
Fig. 6
An 83-year-old woman underwent US for evaluation of an erythematous swelling in the anterior neck and the submandibular space. She had a history of illegal injections into the face and neck to smoothen wrinkles 5 years earlier. She did not initially disclose her previous history of an esthetic procedure. During US, she confirmed her history, and a biopsy was performed.
A. Transverse US of the neck reveals high echogenicity with posterior snow storming (type 1) throughout the subcutaneous fatty layer, and a low echoic nodule with a peripheral hyperechoic rim and posterior enhancement (type 2, arrows).
B. The US-guided gun biopsy specimen shows various vacuoles associated with chronic inflammation, fibrosis, and ill-defined granulomas (hematoxylin and eosin staining, × 100).

Table 1
Clinical Presentations of 39 Patients Who had Undergone Filler Injection in the Cervicofacial Region

| Clinical Presentation | n |
|---|---|
| Asymptomatic | 7 |
| Mass or lump | 18 |
| Migration of the mass | 3 |
| Swelling or edema | 6 |
| Foreign body sensation | 2 |
| Redness or itching | 3 |
| Total | 39 |
Table 2
Types of Filler Injected, Based on Patient Recall

| Type of Injected Filler | n |
|---|---|
| Unknown or illegal material | 24 |
| Collagen | 5 |
| Steroid | 1 |
| Autologous fat or lipocyte stem cell transplantation | 4 |
| Hyaluronic acid | 3 |
| Silicone | 1 |
| Artecol | 1 |
| Total | 39 |
Table 3
Ultrasonographic Features of Complications Associated with the Filler in the Cervicofacial Regions

References
1. Kontis TC, Rivkin A. The history of injectable facial fillers. Facial Plast Surg. 2009; 25:67–72.
2. Goldberg DJ. Breakthroughs in US dermal fillers for facial soft-tissue augmentation. J Cosmet Laser Ther. 2009; 11:240–247.
3. Ginat DT, Schatz CJ. Imaging features of midface injectable fillers and associated complications. AJNR Am J Neuroradiol. 2013; 34:1488–1495.
4. Christensen L, Breiting V, Janssen M, Vuust J, Hogdall E. Adverse reactions to injectable soft tissue permanent fillers. Aesthetic Plast Surg. 2005; 29:34–48.
5. Lombardi T, Samson J, Plantier F, Husson C, Küffer R. Orofacial granulomas after injection of cosmetic fillers. Histopathologic and clinical study of 11 cases. J Oral Pathol Med. 2004; 33:115–120.
6. Dayan SH, Bassichis BA. Facial dermal fillers: selection of appropriate products and techniques. Aesthet Surg J. 2008; 28:335–347.
7. Jham BC, Nikitakis NG, Scheper MA, Papadimitriou JC, Levy BA, Rivera H. Granulomatous foreign-body reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009; 67:280–285.
8. Wortsman X, Wortsman J, Orlandi C, Cardenas G, Sazunic I, Jemec GB. Ultrasound detection and identification of cosmetic fillers in the skin. J Eur Acad Dermatol Venereol. 2012; 26:292–301.
9. Young SR, Bolton PA, Downie J. Use of high-frequency ultrasound in the assessment of injectable dermal fillers. Skin Res Technol. 2008; 14:320–323.
10. Mastruserio DN, Pesqueira MJ, Cobb MW. Severe granulomatous reaction and facial ulceration occurring after subcutaneous silicone injection. J Am Acad Dermatol. 1996; 34(5 Pt 1):849–852.
11. Maly A, Regev E, Meir K, Maly B. Tissue reaction to liquid silicone simulating low-grade liposarcoma following lip augmentation. J Oral Pathol Med. 2004; 33:314.
12. Morhenn VB, Lemperle G, Gallo RL. Phagocytosis of different particulate dermal filler substances by human macrophages and skin cells. Dermatol Surg. 2002; 28:484–490.
13. Shafir R, Amir A, Gur E. Long-term complications of facial injections with Restylane (injectable hyaluronic acid). Plast Reconstr Surg. 2000; 106:1215–1216.
14. Requena C, Izquierdo MJ, Navarro M, Martínez A, Vilata JJ, Botella R, et al. Adverse reactions to injectable aesthetic microimplants. Am J Dermatopathol. 2001; 23:197–202.
15. Verpaele A, Strand A. Restylane SubQ, a non-animal stabilized hyaluronic acid gel for soft tissue augmentation of the mid- and lower face. Aesthet Surg J. 2006; 26:S10–S17.
16. Reda-Lari A. Augmentation of the malar area with polyacrylamide hydrogel: experience with more than 1300 patients. Aesthet Surg J. 2008; 28:131–138.
17. Sturm LP, Cooter RD, Mutimer KL, Graham JC, Maddern GJ. A systematic review of dermal fillers for age-related lines and wrinkles. ANZ J Surg. 2011; 81:9–17.
18. Judodihardjo H, Dykes P. Objective and subjective measurements of cutaneous inflammation after a novel hyaluronic acid injection. Dermatol Surg. 2008; 34:Suppl 1. S110–S114.
19. Lemperle G, Gauthier-Hazan N, Wolters M, Eisemann-Klein M, Zimmermann U, Duffy DM. Foreign body granulomas after all injectable dermal fillers: part 1. Possible causes. Plast Reconstr Surg. 2009; 123:1842–1863.
20. Lemperle G, Rullan PP, Gauthier-Hazan N. Avoiding and treating dermal filler complications. Plast Reconstr Surg. 2006; 118:3 Suppl. 92S–107S.
21. Baumann LS, Halem ML. Lip silicone granulomatous foreign body reaction treated with aldara (imiquimod 5%). Dermatol Surg. 2003; 29:429–443.
22. Rapaport MJ, Vinnik C, Zarem H. Injectable silicone: cause of facial nodules, cellulitis, ulceration, and migration. Aesthetic Plast Surg. 1996; 20:267–276.
23. Hirsch RJ, Stier M. Complications of soft tissue augmentation. J Drugs Dermatol. 2008; 7:841–845.
24. Schelke LW, Van Den Elzen HJ, Erkamp PP, Neumann HA. Use of ultrasound to provide overall information on facial fillers and surrounding tissue. Dermatol Surg. 2010; 36:Suppl 3. 1843–1851.
25. Wortsman X, Wortsman J. Clinical usefulness of variable-frequency ultrasound in localized lesions of the skin. J Am Acad Dermatol. 2010; 62:247–256.
26. Kuwano Y, Ishizaki K, Watanabe R, Nanko H. Efficacy of diagnostic ultrasonography of lipomas, epidermal cysts, and ganglions. Arch Dermatol. 2009; 145:761–764.
27. Fischer J, Metzler G, Schaller M. Cosmetic permanent fillers for soft tissue augmentation: a new contraindication for interferon therapies. Arch Dermatol. 2007; 143:507–510.
28. Feeney JN, Fox JJ, Akhurst T. Radiological impact of the use of calcium hydroxylapatite dermal fillers. Clin Radiol. 2009; 64:897–902.
29. Requena L, Requena C, Christensen L, Zimmermann US, Kutzner H, Cerroni L. Adverse reactions to injectable soft tissue fillers. J Am Acad Dermatol. 2011; 64:1–34. quiz 35-36.
30. Dadzie OE, Mahalingam M, Parada M, El Helou T, Philips T, Bhawan J. Adverse cutaneous reactions to soft tissue fillers--a review of the histological features. J Cutan Pathol. 2008; 35:536–548.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download