INTRODUCTION
Hepatobiliary fascioliasis is a rare zoonotic disease caused by Fasciola hepatica (1). Because its clinical and laboratory findings may be easily confused with other diseases, its radiological findings are important in shortening the diagnostic process (2). Radiographic tools such as ultrasound (US) and computed tomography (CT) are useful not only for diagnosis but also for the follow-up to evaluate the efficacy of the administered therapy (3). In this case, there were unusual findings of multiple aneurysms and active bleeding. We report a case of hepatobiliary fascioliasis with such rare findings for the first time.
CASE REPORT
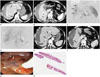
A 52-year-old woman visited our institution with a complaint of upper abdominal pain persisting for the previous two days. Laboratory tests revealed mild leukocytosis (white blood cell count, 12.33 K/µL; normal range, 4-10 K/µL), decreased serum hemoglobin (7.5 g/dL; normal range, 14-18 g/dL), and peripheral blood eosinophilia (25.7% eosinophils, 1950 K/µL; normal range, 1-5% and 50-500 K/µL, respectively). Four-phase CT scans showed multiple ill-defined, hypodense lesions in the peripheral areas of both hepatic lobes. These lesions were observed in all four phases. Some of the lesions were composed of hypoattenuating nodules and were arranged along a line from the periphery to the center of the hepatic lobes. In the arterial phase, active bleeding and subcapsular hematoma were seen in the right hepatic lobe (Fig. 1A, B). The angiography showed multiple aneurysms at the tip of the right hepatic arteries. One of the aneurysms was ruptured, and active bleeding was noted, so transarterial coil embolization was performed to stop the bleeding (Fig. 1C, D). A week after the patient's hospital admission, a follow-up CT was performed. Active bleeding was not found, which verified the migration of the hypodense lesions (Fig. 1E, F). Moreover, the serum hemoglobin increased (11.8 g/dL; normal range, 14-18 g/dL) and the peripheral blood eosinophils decreased (5.3% eosinophils, 670 K/µL). After observation for 1.5 months, hyperbilirubinemia developed (total bilirubin, 4.1 mg/dL; normal range, 0.1-1.2 mg/dL; and direct bilirubin, 3.57 mg/dL; normal range, 0-0.5 mg/dL). Thus, endoscopic retrograde cholangiopancreatography (ERCP) was performed to evaluate the biliary obstruction. The ERCP revealed several moving flat flukes in the common bile duct (Fig. 1G), which were pathologically confirmed as Fasciola hepatica (Fig. 1H).
DISCUSSION
Hepatobiliary fascioliasis is a rare zoonotic disease caused by Fasciola hepatica. Fasciola hepatica usually infects cattle or sheep and humans are accidental hosts (1). Infections occur due to the ingestion of water or raw water plants contaminated with metacercariae, and larvae hatch in the small bowel. Later, the larvae penetrate the bowel wall and migrate through the peritoneal cavity to the liver parenchyma by penetrating the hepatic capsule (2). This period is termed the hepatic (or invasive) stage. It begins one to three months after the initial ingestion. The common signs and symptoms of the disease in the hepatic stage are fever, urticaria, right upper quadrant pain, and marked eosinophilia. Mild hepatitis and severe subcapsular hemorrhage can also be observed (3). In the liver, the flukes gradually tract through the parenchyma and make multiple small holes and cavities, until they reach the larger bile duct and penetrate into the lumen, which is their permanent residence (4). This period is called the biliary stage, when the flukes enter the bile ducts and the gallbladder, where they mature and release eggs. In the biliary stage, the disease usually manifests through intermittent right upper quadrant pain, which may or may not be accompanied by cholangitis and/or cholestasis. Eosinophilia may also be detected (3, 4).
There are several useful methods of diagnosing Fasciola hepatica. Stool studies can be used for ova and parasites, but they are non-diagnostic during the hepatic phase. An enzyme-linked immunosorbent assay is the most widely used method. It is fast, has high sensitivity, and has positive results for all patients regardless of the stage of their disease (3). In most cases, ERCP is not necessary for diagnosis. However, in the clinical presentation, there may be biliary obstructive or pancreatitis-like symptoms and signs that may necessitate ERCP to rule out other possible causes that could produce irregularity and a thickening of the common bile duct wall. As in our case, the flukes can be extracted from the bile ducts. A liver biopsy is not routinely indicated, but it may show eosinophils, histiocytes, granulomas, and in some cases, even eggs (5).
Because the clinical and laboratory findings of fascioliasis may be easily confused with other diseases, its radiological findings are important in shortening the diagnostic process (5). Radiographic tools such as US and CT are useful, not only for diagnosis, but also to evaluate the efficacy of the administered therapy. Although both are useful, US may not be diagnostic in the hepatic phase, because of the heterogeneity of the liver due to poorly defined nodules (3). The flukes migrate gradually through the liver parenchyma and digest hepatocytes. Along the pathway of a fluke, there are multiple small necrotic cavities and abscesses. In the CT scans, they appear as multiple serpentine, branching, subcapsular, and clustered hypoattenuating lesions that point to the central liver (the tunnels and caves sign) (6). Unlike a pyogenic abscess, micro-abscesses caused by Fasciola hepatica cannot coalesce into one large abscess cavity. Since the lesions are not healed through granulation and fibrosis, hepatic necrotic lesions persist for a long time, for months and even years (4). In the biliary stage, the larvae in the bile duct can live for years and move to the larger bile ducts such as the extra-hepatic ducts or the gallbladder. Thus, adult flukes are detected as single or multiple elongated, filamentous, echogenic lesions in US, or as filling defects in a cholangiogram. Spontaneous movement of flukes can be detected in US and cholangiogram. Because adult flukes promote chronic inflammation, the walls of the extra-hepatic ducts and the gallbladder thicken (7).
There have been some reports of fascioliasis accompanied by subcapsular hematoma (8). However, to our knowledge, this is the first report of fascioliasis with multiple aneurysms and active bleeding. When we saw the initial CT scans, we thought that a parasite infection and cholangitic abscesses, which can be seen as ill-defined low-density lesions in the liver, should be considered. But, we also performed angiography and saw multiple aneurysms and active bleeding, which are not usual findings in parasite infections and cholangitic abscesses. Thus, we added vasculitis to our differential diagnosis, which may represent multiple aneurysms, especially polyarteritis nodosa. But, we could not form a definite conclusion until hyperbilirubinemia developed. To evaluate the biliary obstruction, we performed ERCP, which led to the diagnosis of Fasciola hepatica.
Although the exact relationship between Fasciola hepatica and aneurysms of hepatic arteries is unknown, we think Fasciola hepatica causes infectious arteritis and spontaneous rupture. Infected aneurysms can develop from bacteria such as Staphylococcus, Streptococcus, or Escherichia coli, and from fungi such as Aspergillus (9). Through this case, we can consider that Fasciola hepatica caused an infected aneurysm.
Hepatobiliary fascioliasis may typically appear in the form of ill-defined low-density lesions in the peripheral liver, and can sometimes be accompanied by subcapsular hematomas. However, there has been no report yet that parasite infection produces an infected and ruptured aneurysm in the liver. As shown in our case, Fasciola hepatica should be considered even if it is accompanied by multiple aneurysms. We report a case with both the usual radiological findings and the first reported findings of hepatobiliary fascioliasis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download