Abstract
Only a few studies have been reported on the MR contrast enhancement and the apparent diffusion coefficient (ADC) findings of the post-traumatic lesion of the brain. We report a case of the venous ischemia in the left frontal lobe observed in the MRI obtained one day after the incidence of trauma. Considering the presented slight increase in the ADC, the vasogenic edema was thought to be the major mechanism of the venous ischemia and excitotoxic injury. In spite of a slight increase in the ADC, the hyperintensity in the diffusion weighted imaging and contrast-enhanced areas eventually changed into hemorrhagic lesions.
Post-traumatic cerebral ischemia and infarction are the secondary parenchymal injuries with rare incidence caused by the central nervous system traumas (1). The subdural hematoma (SDH) can lead to the post-traumatic ischemia of the brain, and it causes a focal mass effect on the underlying brain parenchyma and decreases the arterial perfusion with the cortical ischemia. Moreover, the SDH can compress the cortical vein and cause venous ischemia of the brain (2, 3).
The mechanism and image findings of both the cerebral venous ischemia and infarction have been reported in the association with the cerebral venous thrombosis. Clinical symptoms and signs of cerebral venous thrombosis vary depending on the location of the occlusion (4). The risk factors of the cerebral venous thrombosis include risks acquired from the conditions such as surgery, trauma, pregnancy, puerperium, antiphospholipid syndrome, cancer, exogenous hormones, and genetic risks such as the hereditary thrombophilia (5).
In this case, radiological findings were obtained two days after the trauma revealed a mass-like lesion in the left frontal lobe, and we considered the possibility of a tumor or traumatic brain lesions. The lesion was diagnosed to be post-traumatic venous ischemia based on the follow-up images. This case report was approved by our Institutional Review Board.
40-year-old female patient visited our institution with seizures, which had developed an hour and half earlier. Generalized tonic-clonic seizure accompanied with the urination and drooling lasted for approximately 30 seconds before stopping; however, the symptoms resumed several minutes later. When she recovered from the seizure, she experienced headache and weakness in the right upper extremity before she decided to come to the emergency room (ER). One day prior to her ER visit, she had hit her head against the waves multiple times while playing water sports. The physical examination conducted upon her arrival at the ER revealed a grade II weakness in her right upper extremity. However, her mental status was alert, and her vital signs were stable.
The brain CT scan revealed a SDH in the left frontal lobe, a 4 × 3 cm2 hypoattenuating lesion at the nearby left frontal lobe and internal petechia (Fig. 1). The brain MRI revealed that the left frontal lobe lesion, which was hypoattenuating on the CT, was located at the superior and middle frontal gyri with hyperintensity on T2-weighted images. When compared to the opposite cerebrum, the cortex of the lesion showed hyperintensity on the diffusion weighted imaging (DWI) and a slight hyperintensity on the apparent diffusion coefficient (ADC) map with contrast enhancement. On the gradient recalled echo image, a focal hemorrhage at the corticomedullary junction was observed. The nearby subcortical white matter of the mass-like lesion revealed edema and partial enhancement (Fig. 2). Therefore, the possibility of a tumor, such as lymphoma and metastasis, was considered, and differential diagnoses from the post-traumatic venous ischemia, post-traumatic vasospasm, and cerebral contusion were performed. The SDH was confirmed in the left frontal lobe area. Furthermore, the left wall and lumen of the anterior superior sagittal sinus close to the lesion showed an asymmetric enhancement in the contrast-enhanced coronal images.
Craniotomy and removal of the SDH were performed. In the surgical findings, the bridging vein was torn, and approximately 100 cc of hematoma was removed. After eight days, the postoperative brain CT images revealed the aggravation of the brain edema in the left frontal lobe without any significant change in the focal hemorrhage.
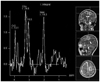
The brain MRI obtained 19 days postoperatively the MR spectroscopy of the frontal lobe lesion, revealed hemorrhage of the left frontal cortex and a remaining edema similar to that of the 8th day postoperative CT image. The MR spectroscopy showed that the Choline (Cho)/Creatinine (Cr) ratio increased to 1.33, and the Cho/N-acetyl aspartate ratio increased to 1.62 (Fig. 3). Accordingly, the possibility of a tumor could not be excluded.
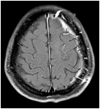
Weakness of the upper extremity improved after the conservative treatments including rehabilitation, and the patient was discharged. A two-months follow-up brain MRI revealed that the size of the left frontal lobe lesion significantly decreased, and other associated findings mostly improved (Fig. 4). Finally, she was diagnosed with the post-traumatic venous ischemia.
In this case, the patient, with clinical symptoms of traumatic brain damage, underwent an initial brain MRI, which showed the hyperintensity of the left frontal superior and middle gyral cortices on the DWI with associated contrast enhancement and petechia in the corticomedullary junction. These findings complicated the differential diagnosis.
The one-week follow-up the brain CT revealed that the hemorrhage did not advance, and no additional hematoma was observed. Accordingly, the possibility of a cerebral contusion was thought to be low. In the case of the cerebral contusion, hemorrhage usually tends to aggravate 24-48 hours after the onset or additional hemorrhage develops, even though the initial CT images may show normal findings. In the case of the contusional hemorrhage, the abnormal petechia and edema were observed in the surrounding areas of the nearby calvaria, along the gyral crest (6). Usually, hemorrhages occur in the cortex and subcortical white matter in a cerebral contusion. However, two petechial lesions were observed at the corticomedullary junction, not at the cortex, in this case. Accordingly, a hemorrhagic parenchymal change might be caused by the diffuse axonal injury or venous congestion.
The possibility of a post-traumatic vasospasm was also considered in this case. Post-traumatic vasospasm are observed in the patients with traumatic subarachnoid hemorrhage (SAH), intraventricular hemorrhage, SDH, or contusion, which are frequently observed in the patients with a small parenchymal contusion of 1 cm or less (7). Post-traumatic vasospasm can develop from 12 hours to 5 days after the trauma in the patients without traumatic SAH, because of the direct stretching and mechanical irritation of the cerebral artery in the contusion area (8).
In the case of arterial occlusive lesion caused by the post-traumatic vasospasm, there was mild vasogenic edema, and no contrast enhancement two days after the injury was observed. However, there were no such findings in the case of this patient. Considering the image findings and their timing, the case evidence did not correspond to the findings of acute infarction caused by a post-traumatic vasospasm.
In the MRI obtained on the 19th day of the admission for the differentiation of the tumor from the venous infarction, a hemorrhage developed in the left frontal cortex, and an edema in the nearby white matter had deteriorated relative to that seen in the initial images. The MR spectroscopy revealed that the Cho/Cr ratio of the lesion slightly increased to 1.33; therefore, the possibility of a tumor could not be excluded. In MR spectroscopy, Cho is known to increase when the neural cell membranes are destroyed or biosynthesis is activated. The increase in Cho is particularly abrupt in the case of a malignant brain tumor. The Cho/Cr cut-off value, which can determine whether the tumors are benign or malignant, is known to be 1.84 when the 144 msec of intermediate echo time is used (9). The Cho/Cr of this case was 1.33, which did not clearly imply the presence of a malignant tumor, and the upper extremity weakness was improving. As a result, follow-up brain imaging instead of a biopsy was performed.
While the subject patient was playing water sports, she hit her head against the water several times, resulting in a SDH in her left frontal lobe area. Moreover, the venous pressure in the nearby frontal lobe increased to destroy the blood-brain barrier (BBB), and she developed a vasogenic edema, which eventually converted into a venous ischemia (1).
The cortical veins of the superior frontal gyrus and middle frontal gyrus, where the lesion was located, were connected with the anterior superior sagittal sinus. An MR venography was not performed, but the thrombus in the cortical vein in the left frontal lobe might have become a hindrance because of the SDH. It was impossible to confirm the presence of thrombus; however, considering the asymmetric contrast enhancement of the left wall and lumen of the anterior superior sagittal sinus close to the lesion, there might have been a venous flow dysfunction in the left frontal lobe. Once venous congestion develops, prominent cortical venous enhancement and leptomeningeal enhancement may follow (10).
In the previous studies on the cerebral venous infarction caused by the venous sinus thrombosis, the ADC level decreased two days after the symptoms appeared and then gradually increased. In the early stage of the cerebral venous infarction, cytotoxic edema developed first. Soon, the ADC level increased due to vasogenic edema, and the T2 hyperintensity appeared (1). In this case, the MRI was obtained the next day after the trauma, and on the same day of the seizure development. The slight increase in the ADC level as well as the hyperintensity observed on the T2-weighted images, implied that the vasogenic edema was the major mechanism for the venous ischemia presented in this case. It is possible for the vasogenic edema to abruptly deteriorate, which may offset the ADC value, reflecting the initial cytotoxic edema. However, the possibility was very low considering that the time for this process was too short to be of significance. When the ADC decreases, the parenchymal sequelae will remain, but when the ADC is in a normal level or increases, the lesion is known to recover (10). In this case, the MRI findings of the slightly increased ADC level, hyperintensity on the DWI, and the contrast-enhancement mainly of the cortex could imply an additional damage to the brain because of the seizure. The edema in the subcortical white matter regressed; however, a hemorrhage in the cortex eventually developed.
This is an example of a difficult recovery of a post-traumatic venous ischemia with the hyperintensity on the DWI and the destruction of the BBB without regard to the slightly increased ADC level.
Venous ischemia from the venous congestion often shows prominent vasogenic edema with mass effect. However, this case didn't show marked mass effect on the MRI finding in comparison to the size of the lesion. Therefore, we considered a possibility of excitotoxic injury. The limitation of our study was not performing the MR venography, because of uncertainty of thrombus in the cortical vein around left frontal lobe lesion, due to SDH, was the limitation of this study.
Figures and Tables
Fig. 1
Initial (A) and 8-days follow-up (B) axial CT images.
A. Initial axial CT image shows subdural hematoma and brain swelling in the left frontal lobe with focal hyperattenuated spot, suggesting hemorrhage.
B. Eight-days follow-up axial CT image shows progressive white matter edema in the left frontal lobe without gross change that suggests focal hemorrhage in the corticomedullary junction.
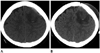
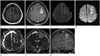
Fig. 2
A 40-year-old woman with acute trauma and seizure.
A. Axial T1-weighted MR image shows hypointensity on the left frontal lobe.
B. Axial gadolinium-enhanced T1-weighted MR image shows contrast-enhancement of the left frontal lobe.
C. Axial gradient-echo MR image shows two hypointensities in the corticomedullary junction of the left frontal lobe, suggesting hemorrhage.
D. Axial diffusion-weighted MR image shows cortical hyperintensity in the left frontal lobe.
E. Axial apparent diffusion coefficient MR image shows slight cortical hyperintensity in the left frontal lobe with edema of adjacent white matter.
F. Coronal T2-weighted MR image shows hyperintensity in the superior and middle frontal gyrus with edema of subcortical white matter.
G. Coronal gadolinium-enhanced T1-weighted MR image shows gyral enhancement of the left frontal lobe. Asymmetric high signal intensity in the left side wall and lumen of the anterior superior sagittal sinus is seen.
References
1. Matsushige T, Nakaoka M, Kiya K, Takeda T, Kurisu K. Cerebral sinovenous thrombosis after closed head injury. J Trauma. 2009; 66:1599–1604.
2. Forbes KP, Pipe JG, Heiserman JE. Evidence for cytotoxic edema in the pathogenesis of cerebral venous infarction. AJNR Am J Neuroradiol. 2001; 22:450–455.
3. Osborn AG. Osborn's barin: imaging, pathology and anatomy. Salt Lake City: Amirsys;2013. p. 1–72.
4. Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005; 352:1791–1798.
5. Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011; 42:1158–1192.
6. Alahmadi H, Vachhrajani S, Cusimano MD. The natural history of brain contusion: an analysis of radiological and clinical progression. J Neurosurg. 2010; 112:1139–1145.
7. Armin SS, Colohan AR, Zhang JH. Vasospasm in traumatic brain injury. Acta Neurochir Suppl. 2008; 104:421–425.
8. Shahlaie K, Keachie K, Hutchins IM, Rudisill N, Madden LK, Smith KA, et al. Risk factors for posttraumatic vasospasm. J Neurosurg. 2011; 115:602–611.
9. Kim JH, Chang KH, Na DG, Song IC, Kwon BJ, Han MH, et al. 3T 1H-MR spectroscopy in grading of cerebral gliomas: comparison of short and intermediate echo time sequences. AJNR Am J Neuroradiol. 2006; 27:1412–1418.
10. Leach JL, Fortuna RB, Jones BV, Gaskill-Shipley MF. Imaging of cerebral venous thrombosis: current techniques, spectrum of findings, and diagnostic pitfalls. Radiographics. 2006; 26:Suppl 1. S19–S41. discussion S42-S43.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download