Abstract
Ewing's sarcoma presents a rare tumor of the head and neck, and even rarer in the nasal cavity and/or paranasal sinuses. We report the case of Ewing's sarcoma in the nasal cavity, as presented with nasal obstruction and epistaxis. The CT and MRI examination reveals a mass in the left nasal cavity with extension to contralateral side, ethmoidal sinus, and nasopharynx. We provide an overview of Ewing's sarcoma in the nasal cavity and discuss radiologic findings of this unusual case.
Ewing's sarcoma is a highly malignant tumor found mainly in children and young adults, most commonly arising from skeletal structures, especially in long bones. Primary Ewing's sarcoma of the head and neck is very rare, accounting for only 2-3% of all Ewing's sarcomas, and even rarer in the nasal cavity and/or paranasal sinuses (1, 2).
Ewing's sarcoma of the long bone is known to be a well-enhancing soft tissue density mass without calcification on the CT examination. On the MRI examination, it is known to be hypointense to isointense on T1 weighted imaging (WI) and various signals intense on T2WI with heterogeneously marked enhancement by gadolinium. Invasions of subcutaneous tissue and ill-defined bony destructive changes are common (1, 3-5).
We provide an overview of Ewing's sarcoma and present a case of the Ewing's sarcoma in the nasal cavity.
A 42-year-old male visited the otorhinolaryngology department for nasal obstruction, epistaxis and intermittent rhinorrhea for 2 months. At the endoscopic examination, a lobulated mass coated with discharge was detected in the left nasal cavity expanding to contralateral side (Fig. 1).
Pre-contrast and post-contrast CT revealed a 2.5 × 4.0 × 3.5 cm sized mass in the left nasal cavity. The lesion was extended to contralateral side with bony septal destruction, and hard palate was also destroyed by inferior extension of the mass. It was expanded to the left posterior ethmoidal sinus and posteriorly reached up to the anterior border of nasopharynx. The mass had soft tissue density with absence of calcification. The mass showed heterogeneous enhancement after intravenous bolus injection of contrast media (Fig. 2A, B).
The MRI examination was performed on 1.5 T using a Siemens Avanto equipment. On T2WI, an expansile lobulated mass in the left nasal cavity showed hyperintense signal intensity compared with the muscle (Fig. 2C). On T1WI, the mass showed slight hyperintense signal intensity (Fig. 2D, E). Postcontrast T1-weighted image showed heterogeneous enhancement of the mass. As seen on CT scan, extension to adjacent structures was also being detected (Fig. 2F). Metastatic lymphadenopathy was not seen.
The mass in the nasal cavity with extensive invasion into adjacent structures and bony destructive change suggests malignant nature of the tumor. Thus, our differential radiological diagnoses of this lesion were malignant lymphoma, rhabdomyosarcoma and poorly-differentiated carcinoma.
An endoscopic wide excision was performed under general anaesthesia. According to operative findings, a submucosal mass in the left nasal cavity with obstruction of the left choana was noted. Destruction of posterior portion of nasal septum was also detected.
Grossly, the specimens were irregularly fragmented myxoid and necrotic mucosal tissues. On microscopic examination, the tumor was composed of densely distributed, uniform, small- to medium-sized, round cells with scanty cytoplasm (Fig. 3A). The microscopic diagnosis was malignant neoplasm. The tumor cells were immunoreactive with Friend leukemia integration-1 (FLI-1) protein (Fig. 3B). The immunohistochemistric diagnosis was consistent with Ewing's sarcoma. Molecular studies using polymerase chain reaction (PCR) confirmed EWS-FLI-1 [t(11;22) (q24;q12)].
Distant metastasis was excluded by fluorodeoxyglucose positron emission tomography (not shown). After endoscopoic excision, the patient was treated with chemotherapy, which consisted of 4 cycles of vincristine, actinomycin D and cyclophosphamide. On a follow-up endoscopy performed 106 days after operation, tumor recurrence was not detected.
Ewing's sarcoma is highly malignant, small, round cell tumor, originated from primitive neuroectodermal cells, as first described by James Ewing in 1921. Primary Ewing's sarcoma is represented commonly in early childhood, adolescence but rarely in adulthood. There is slightly male predominance as 1.5 : 1 male to female ratio (4, 6, 7).
Ewing's sarcoma is distinguished by two types: skeletal type and extraskeletal type. Most commonly, Ewing's sarcoma arises from skeletal structures, especially long bones (35%), and pelvis (24%). The extraskeletal type of Ewing's sarcoma usually occurs in the soft tissue of the lower extremities and the paravertebral region (8).
Thirteen cases of primary Ewing's sarcoma in the nasal cavity and/or paranasal sinuses have been reported in the otolaryngology literature (1, 7, 9). In the radiology literature, only a single case report from 2003 by Harman et al. (3) has been described.
The prognosis for Ewing's sarcoma depends on the presence of metastases, because Ewing's sarcoma is highly malignant and metastasizes early to bones and lungs. Recently, it is accepted that prompt chemotherapy is necessary to treat occult metastasis, and a combination of surgical excision, radiotherapy and chemotherapy has significantly improved the 5-year survival rates, now reaching to 75% (1, 9).
Microscopically, Ewing's sarcoma shows uniform, small, round cells with round to elongated nuclei, scanty cytoplasms and indistinct cytoplasmic borders. Hemorrhagic areas and extensive necrotic lesions are common. The essential diagnostic examination for Ewing's sarcoma among many small round neoplasms is CD99 (O13) marker, the specific immunohistochemical examination. In addition, molecular studies using PCR to detect characteristics of chromosomal translocations are definitive for the diagnosis of Ewing's sarcoma. Specific genetic hallmarks of Ewing's sarcoma is a gene sequence t(11;22)(q24;q12), which results in the fusion of the EWS gene with the FLI gene (1, 9, 10).
On the CT examination, Ewing's sarcomas show similar findings regardless of the primary site. It is known to be a well-enhancing, soft tissue density mass without calcification. If a tumor includes hemorrhage or necrosis, it may be detected as heterogeneous pattern. Invasions of subcutaneous tissues and ill-defined bony destructive changes are common, and these reflect the aggressive nature of the tumor (3, 5)
The MRI examination is more effective than the CT scan in determining the nature of tumors, delineating tumor margins, evaluating soft tissue involvement, and determining tumor stage and operative approach. On the MRI examination, Ewing's sarcoma in the nasal cavity is known to be hypointense to isointense on T1WI and varying signal intense on T2WI. It is heterogeneously and markedly enhanced by gadolinium (3, 5).
In our study, the expansile lobulated mass in the left nasal cavity showed soft tissue density and inhomogeneous enhancement without calcification or cystic component on the CT scan. On the MR imaging, the lesion showed slightly hyperintense signal relative to the muscles on T1WI, and hyperintense signal on T2WI with heterogenous enhancement. Extension to contralateral nasal cavity, ethmoidal sinus, nasopharynx and hard palate was detected with extensive bony destructive change.
These findings suggest malignant nature, and differential radiological diagnoses should be included malignant lymphoma, rhabdomyosarcoma, poorly-differentiated carcinomas and Ewing's sarcoma. These tumors share radiologic findings and it is hard to conclude specific diagnosis without more examinations (1, 8).
In conclusion, primary Ewing's sarcoma must be considered, when the expansile nasal mass is detected with extensive invasion into adjacent structures and bony destructive changes, although which is a very rare condition. Realizing the nature of Ewing's sarcoma and understanding its diagnostic significance can lead to the approach of appropriate management.
Figures and Tables
Fig. 1
Initial endoscopic features. A lobulated mass (arrows) coated with discharge was detected in the left nasal cavity expanding to contralateral side.
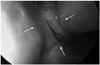
Fig. 2
Ewing's sarcoma in the left nasal cavity in a 42-year-old man. Postcontrast coronal CT image (A) shows an expansile and well-defined soft tissue mass (arrows) in the left nasal cavity with inhomogeneous enhancement. The mass extends to the contralateral nasal cavity, left posterior ethmoid sinus and hard palate. Coronal CT image with bone algorithm (B) demonstrates better bony destructive change. On precontrast coronal T2-weighted image with fat suppression (C), an expansile lobulated mass (arrows) in the left nasal cavity shows hyperintense signal intensity compared with the muscle. Precontrast coronal and axial T1-weighted images with fat suppression (D, E) show slightly hyperintense signal intensity. Postcontrast T1-weighted axial image with fat suppression (F) reveals heterogeneous enhancement of the mass. As seen on CT images, extension to adjacent structures with bony destructive change is also noted.
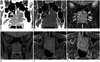
Fig. 3
Pathologic features of Ewing sarcoma/Primitive neuroectodermal tumor.
A. The tumor is composed of densely distributed, uniform, small to medium sized, round cells with scanty cytoplasm. Mitotic figures are readily found (Hematoxylin & Eosin, × 400).
B. The tumor cells are immunoreactive with FLI-1 protein (Immunohistochemistry, × 400).
Note.-FLI-1 = Friend leukemia integration-1

References
1. Kawabata M, Yoshifuku K, Sagara Y, Kurono Y. Ewing's sarcoma/primitive neuroectodermal tumour occurring in the maxillary sinus. Rhinology. 2008; 46:75–78.
2. Aferzon M, Wood WE, Powell JR. Ewing's sarcoma of the ethmoid sinus. Otolaryngol Head Neck Surg. 2003; 128:897–901.
3. Harman M, Kiroğlu F, Kösem M, Unal O. Primary Ewing's sarcoma of the paranasal sinus with intracranial extension: imaging features. Dentomaxillofac Radiol. 2003; 32:343–346.
4. Howarth KL, Khodaei I, Karkanevatos A, Clarke RW. A sinonasal primary Ewing's sarcoma. Int J Pediatr Otorhinolaryngol. 2004; 68:221–224.
5. Javery O, Krajewski K, O'Regan K, Kis B, Giardino A, Jagannathan J, et al. A to Z of extraskeletal Ewing sarcoma family of tumors in adults: imaging features of primary disease, metastatic patterns, and treatment responses. AJR Am J Roentgenol. 2011; 197:W1015–W1022.
6. Csokonai LV, Liktor B, Arató G, Helffrich F. Ewing's sarcoma in the nasal cavity. Otolaryngol Head Neck Surg. 2001; 125:665–667.
7. Yeshvanth SK, Ninan K, Bhandary SK, Lakshinarayana KP, Shetty JK, Makannavar JH. Rare case of extraskeletal Ewings sarcoma of the sinonasal tract. J Cancer Res Ther. 2012; 8:142–144.
8. Coskun BU, Cinar U, Savk H, Basak T, Dadas B. Isolated maxillary sinus Ewing's sarcoma. Rhinology. 2005; 43:225–228.
9. Gupta S, Gupta OP, Mehrotra S, Mehrotra D. Ewing sarcoma of the maxilla: a rare presentation. Quintessence Int. 2009; 40:135–140.
10. Iezzoni JC, Mills SE. "Undifferentiated" small round cell tumors of the sinonasal tract: differential diagnosis update. Am J Clin Pathol. 2005; 124:S110–S121.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download