INTRODUCTION
Due to the remarkable advances in the treatment of prostate cancer (Pca), a large proportion of patients are diagnosed with loco-regional disease and have excellent cancer-related survival.
1 Men with Pca, however, have higher non cancer mortality rates than the general population, and some of these excess deaths may be related to treatment.
2 There are many reports of the adverse effects of Pca treatment, especially androgen deprivation therapy (ADT), that could potentially impact overall health status and affect the mortality rate.
Although ADT has been a treatment of choice for patients with metastatic disease since it was introduced in the 1940s, evidence from randomized trials supports the use of ADT in combination with external-beam radiation therapy for locally advanced Pca with high-risk features.
34 Even localized disease without adverse pathologic features (pT stage T2b or less) has been shown to benefit from combination therapy with ADT,
5 accounting for a dramatic increase in ADT use.
6 Despite its excellent treatment effects, ADT is associated with numerous adverse effects, including vasomotor flushing, osteoporosis, fatigue, loss of libido, and gynecomastia.
78 Patients may develop more serious events, including cardiovascular disease, and diabetes mellitus (DM), which in turn can lead to significant decrease in quality of life or even life-threatening consequences.
There are some controversial reports about these notable adverse effects. Several previous studies reported that ADT was associated with increased risks of myocardial infarction (MI) along with earlier onset of fatal MI.
1910 In contrast to these findings, there are many reports that suggest that the association between ADT use and these serious events is questionable. A report from the Radiation Therapy Oncology Group protocol 86-10 found that neoadjuvant ADT was not associated with an increased risk of cardiac events.
11 Adjuvant ADT was also not associated with increased risk of cardiovascular mortality.
12 Taken together, these results have collectively led to significant uncertainty in clinical practice. Because ADT with a gonadotropin-releasing hormone agonist (GnRHa) continues to be a crucial component of the treatment of Pca, identifying potential risks related to GnRHa use is important. We conducted this population-based study to investigate whether GnRHa use in patients with Pca was associated with increased incidences of cardiovascular diseases.
RESULTS
During the follow-up, 441 (9.4%) and 151 (3.2%) patients were newly diagnosed with CVA and IHD, respectively. The mean age of the cohort at diagnosis was 69.3 ± 9.2 years and the mean duration of GnRHa use was 11.1 ± 17.4 months. Compared with GnRHa users, nonusers were significantly younger (users, 72.6 ± 8.3, and nonusers, 66.8 ± 9.0 years;
P < 0.001), more likely to reside in the urban area (users, 75.6% and nonusers, 79.7%;
P = 0.002), and more likely to have National Health Insurance rather than be supported by the Medicaid system (users, 94.2%, and nonusers, 96.9%;
P < 0.001). The two groups did not differ significantly with regard to previous medical histories that might have an impact on the development of cardiovascular disease (
Table 1). However, the rate of exposure to related medications was significantly higher in nonusers than in users, except for antiplatelet therapy. Furthermore, although there were no differences between the groups in previous medical conditions that was included in the analysis, the calculated comorbidity score using the CCI scoring system showed a significant difference in favor of nonusers (
Table 1).
Table 1
Basic characteristics of GnRHa users and nonusers

|
Characteristics |
GnRHa users (n = 2,053) |
GnRHa nonusers (n = 2,654) |
P value |
|
Age at diagnosis, yr |
|
|
< 0.001 |
|
< 55 |
53 (2.6) |
246 (9.3) |
|
55–64 |
277 (13.5) |
785 (29.6) |
|
65–74 |
806 (39.2) |
1,107 (41.7) |
|
≥ 75 |
917 (44.7) |
516 (19.4) |
|
Residence |
|
|
0.002 |
|
Urban |
1,552 (75.6) |
2,116 (79.7) |
|
Suburban/rural |
501 (24.4) |
538 (20.3) |
|
Insurance type |
|
|
< 0.001 |
|
NHI |
1,933 (94.2) |
2,571 (96.9) |
|
Medicaid |
120 (5.8) |
83 (3.1) |
|
Prior medication use |
|
|
|
|
Statin |
140 (6.8) |
219 (8.3) |
0.035 |
|
Antihypertensive |
203 (9.9) |
318 (12.0) |
0.017 |
|
Anticoagulant |
185 (9.0) |
348 (13.1) |
< 0.001 |
|
Antiplatelet |
91 (4.4) |
101 (3.8) |
0.457 |
|
Prior antiandrogen use |
330 (16.0) |
0 (0.0) |
- |
|
Medical history |
|
|
|
|
Hypertension |
896 (43.6) |
1,126 (42.4) |
0.344 |
|
DM |
454 (22.1) |
592 (22.3) |
0.420 |
|
Liver disease |
56 (2.7) |
89 (3.4) |
0.112 |
|
Other cancer |
243 (11.8) |
280 (10.6) |
0.211 |
|
Chronic kidney disease |
52 (2.5) |
48 (1.8) |
0.079 |
|
COPD |
188 (9.2) |
252 (9.4) |
0.767 |
|
Asthma |
158 (7.7) |
176 (6.6) |
0.135 |
|
Peripheral vascular disease |
220 (10.7) |
277 (10.4) |
0.679 |
|
Other treatment |
|
|
< 0.001 |
|
Radical prostatectomy |
0 (0.0) |
1,603 (60.4) |
|
Radiotherapy |
458 (22.3) |
517 (19.5) |
|
Charlson comorbidity index |
|
|
< 0.001 |
|
0, 1 |
116 (5.7) |
414 (15.6) |
|
2 |
341 (16.6) |
713 (26.9) |
|
3 |
687 (33.5) |
772 (29.1) |
|
4 |
510 (24.8) |
431 (16.2) |
|
≥ 5 |
399 (19.4) |
328 (12.4) |
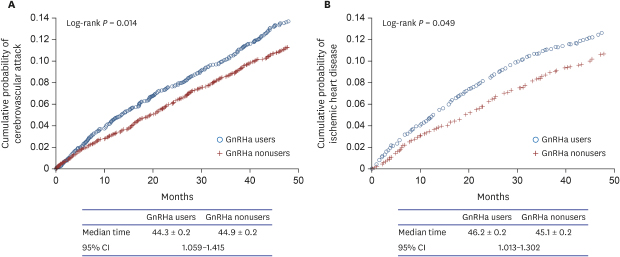
Kaplan-Meier curves for each of the outcomes—CVA and IHD—as univariate analyses are shown in
Fig. 1. GnRHa users were more likely than nonusers to develop CVA and IHD (log rank,
P = 0.013 and 0.048, respectively). The median time from the start of follow-up to the development of CVA was 44.3 ± 0.2 (standard error) months for GnRHa users and 44.9 ± 0.2 months for nonusers with 95% confidence interval of 1.059 to 1.415. However, in the multivariate analysis with Cox proportional hazards models, we found that GnRHa use was not independently associated with development of CVA and IHD (
Table 2). Important significant predictors of CVA and IHD development were age as a continuous variable, prior hypertension, DM, chronic obstructive pulmonary disease, and CCI as a continuous variable (
Table 2). Treatment modalities including radical prostatectomy and/or radiotherapy were not associated with the outcomes. Independent predictors of each disease outcome are shown in
Table 2.
Fig. 1
Kaplan-Meier curves for the outcomes comparing GnRHa users to nonusers. (A) cerebrovascular attack, (B) ischemic heart disease.
GnRHa = gonadotropin-releasing hormone agonist, CI = confidence interval.

Table 2
Multivariate Cox proportional hazard model predicting risk of cerebrovascular attack, IHD, MI, and DM
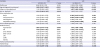
|
Variables |
CVA |
IHD |
|
HR (95% CI) |
P value |
HR (95% CI) |
P value |
|
GnRHa use |
0.926 (0.791–1.111) |
0.359 |
0.931 (0.785–0.186) |
0.135 |
|
Age at diagnosis (continuous) |
1.069 (1.057–1.095)
|
< 0.001
|
1.039 (1.019–1.010)
|
< 0.001
|
|
Urban vs. suburban/rural |
1.221 (0.958–1.498) |
0.063 |
1.617 (1.305–2.028)
|
< 0.001
|
|
NHI vs. Medicaid |
1.704 (1.198–2.307)
|
0.002
|
1.342 (0.931–2.012) |
0.098 |
|
Prior medication use |
|
|
|
|
|
Statin |
1.264 (0.953–1.676) |
0.104 |
0.395 (0.497–0.915)
|
0.021
|
|
Antihypertensive |
1.064 (0.923–1.598) |
0.099 |
0.826 (0.628–0.986)
|
0.037
|
|
Anticoagulant |
0.907 (0.696–1.182) |
0.470 |
0.986 (0.758–1.193) |
0.895 |
|
Antiplatelet therapy |
1.317 (0.917–1.891) |
0.135 |
0.710 (0.458–0.965)
|
0.009
|
|
Prior antiandrogen use |
0.897 (0.548–1.015) |
0.088 |
0.812 (0.595–1.152) |
0.128 |
|
Medical history |
|
|
|
|
|
Hypertension |
1.200 (1.019–1.412)
|
0.028
|
1.721 (1.398–1.998)
|
< 0.001
|
|
DM |
1.302 (1.023–1.521)
|
0.017
|
1.652 (1.345–2.003)
|
< 0.001
|
|
Liver disease |
1.150 (0.744–1.778) |
0.530 |
1.329 (0.902–2.025) |
0.386 |
|
Other cancer |
1.206 (0.982–1.482) |
0.074 |
0.986 (0.605–1.152) |
0.098 |
|
CKD |
0.876 (0.483–1.592) |
0.665 |
1.425 (0.898–2.197) |
0.197 |
|
COPD |
1.427 (1.115–1.826)
|
0.005
|
1.398 (1.108–1.912)
|
0.003
|
|
Asthma |
1.232 (0.920–1.651) |
0.161 |
1.205 (0.831–1.612) |
0.295 |
|
Radical prostatectomy |
1.205 (0.865–2.057) |
0.395 |
1.207 (0.776–1.912) |
0.562 |
|
Radiotherapy |
1.198 (0.698–2.157) |
0.385 |
1.386 (0.837–2.514) |
0.473 |
|
CCI (continuous) |
1.051 (1.009–1.112)
|
0.002
|
1.038 (1.021–1.103)
|
0.002
|
To investigate the impact of duration of GnRHa use on the development of cardiovascular disease, we performed further analyses by categorizing the cohort into four groups according to duration of GnRHa use: non users, 12 months or less, 13 to 24 months, 25 to 36 months, and 37 months or more. In multivariate analysis, GnRHa use was still non associated with both outcomes, and increasing duration of use was not associated with increased risk of cardiovascular disease (
Table 3). In contrast, age and previous diagnosis of hypertension remained as independent predictors of the development of CVA and IHD (data not shown).
Table 3
Association between duration of GnRHa use and cerebrovascular attack, IHD, MI, and DMa

|
Variables |
CVA |
IHD |
|
HR |
95% CI |
HR |
95% CI |
|
Duration of GnRHa use, mon |
|
|
|
|
|
No use |
Reference |
|
Reference |
|
|
≤ 12 |
1.302 |
0.910–1.895 |
0.654 |
0.517–1.685 |
|
13–24 |
0.982 |
0.598–1.180 |
1.194 |
0.752–1.594 |
|
25–36 |
1.652
|
1.021–2.025
|
1.359 |
0.845–1.726 |
|
≥ 37 |
1.215 |
0.877–1.524 |
1.217 |
0.612–1.783 |
|
P value |
0.090 |
0.139 |
DISCUSSION
There has been great interest in the adverse cardiovascular effects of ADT, given the suggestions that low serum testosterone level is associated with coronary artery disease.
1415 Several studies have reported an association between ADT and increased risk of developing cardiovascular disease. A claims-based analysis of more than 70,000 Pca patients reported that GnRHa use increased the risk of incident DM, coronary heart disease, MI, and sudden cardiac death.
1 A report based on the Surveillance, Epidemiology, and End Results (SEER)-Medicaid database found that patients who received ADT for at least 1 year had a 20% higher risk of cardiovascular morbidity than control patients.
16 Another study using linked administrative databases showed that men aged 66 years or older with Pca who were given at least 6 months of ADT had an increased risk of DM,
1718 but not of MI or sudden cardiac death.
19 Thus, the findings of studies of the association between ADT and cardiovascular disease that have suggested a positive correlation remain somewhat mixed, making the issue quite confusing.
The above-mentioned studies have a few weaknesses. First, most studies did not randomly assign the patients either to ADT or to s control group. This of course makes it possible that factors related to ADT might also be related to cardiovascular disease. Second, these studies might have overlooked the possibility that patients receiving regular prescriptions of ADT visit the hospital more frequently, which would make them more likely to be diagnosed with diseases of interest.
1 Third, different studies focused on different outcomes. For example, some studies analyzed the incidence rates, while others investigated disease-related morbidity or mortality.
11619
Although our study may have similar limitations, our results are based on the entire Korean population, which included all Pca patients from the whole nation during the study period, rather than sampling a certain range of the population (e.g., database from Medicaid or from different insurers), as was done in the previous studies.
119 Analyzing the patient cohort from the whole nation may have reduced possible bias that might have resulted from patient cohort sampling. In this nation-wide, population-based study of men with Pca, we found that ADT with GnRHa was not associated with increased risks of cardiovascular disease. The result did not change when the duration of GnRHa use was taken into consideration. These results were confirmed after accounting for oral antiandrogen use and additional clinical information, such as age at diagnosis, medications and previous diagnoses that might have been related to the outcomes of interest, and SES. Our analysis found that the important factors related to increased risk of developing cardiovascular disease were the patient’s age and previous diagnosis of hypertension and/or DM.
Several studies reported results supporting our findings. Studies investigating morbidity related to cardiovascular events had demonstrated similar results,
1619 and as for mortality, a few prior randomized trials had found that there was no association between ADT and cardiovascular mortality.
1112
We hypothesized that the main reason that ADT use seemed to be related to cardiovascular disease in previous studies was discrepancies in patient characteristics between the ADT and control groups. This might have caused a few defects in controlling for confounding factors in the analysis. As seen in our data, GnRHa nonusers tended to be younger, reside more in urban areas, and have higher SES (fewer included in Medicaid). Notably, although there were no differences in past medical history between GnRHa users and nonusers, a significant difference was observed in prior use of relevant medications (
Table 1). This suggests that nonusers may visit medical facilities more often and receive more appropriate treatment for their health issues, which can greatly affect the development of cardiovascular disease. Furthermore, nonusers had significantly less comorbidity than users as calculated by the CCI. This indicates that nonusers were healthier than users from the beginning, which could lead to the misleading conclusion that GnRH use is associated with major health problems. Our hypothesis is further supported by the current finding that most of the variables that were shown to predict the development of either CVA or IHD independently in the multivariate analysis, such as age at diagnosis, SES, and CCI (
Table 2), also differed significantly between GnRHa users and nonusers (
Table 1). Therefore, our findings can be interpreted as indicating that factors such as age, SES, and comorbidities, and not ADT, may have affected the development of the diseases of interest.
The current study has a few drawbacks. Because of the limitations of the HIRA database, which does not include data on tumor characteristics, such as tumor stage and Gleason score, the effects of the tumor itself on cardiovascular diseases and DM could not be identified. All patients in our study had relatively short follow-up period of around 4 years, and further analysis of long-term results is required. We also did not include mortality data, and therefore care should be taken when applying these results to the clinical setting. Furthermore, although radical prostatectomy was included in the analysis and found to be not associated with the outcome, there may still be a remaining risk of bias masking the effect of GnRHa on the outcome. The effect of major surgery should be refined in the future study. The most important advantage of the current study is that it included the entire Korean population of Pca patients during the study period, because the entire Korean population is covered by either National Health Insurance or Medicaid (approximately 97% and 3%, respectively). All patients in this study were diagnosed during the same 6-month period, started follow-up at a similar time (from July 1, 2012 until December 31, 2012), and completed follow-up at the same time (December 31, 2016) to minimize confounding factors as it is in the prospective study.
In conclusion, ADT using GnRHa did not seem to increase the risk of developing cardiovascular disease. The increased incidence of such diseases seen in men with ADT is believed to be due to inequalities in patient characteristics, including age, SES, and comorbidities. However, our result does not exclude the possibility that ADT increases mortality related to cardiovascular adverse effects, because we could not analyze overall survival. Further randomized, long-term studies are warranted to establish strategies for ADT use in the clinical setting, especially in patients at high risk for developing cardiovascular disease.








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download