DISCUSSION
Previous studies have indicated that the most common intracranial abnormalities associated with CPP are hypothalamic hamartoma, glioma, astrocytoma, and pinealoma.
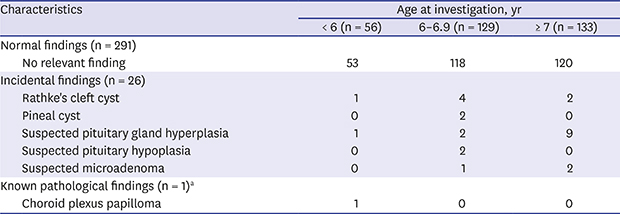
11 However, the present study did not detect any pathological lesions among girls with CPP, regardless of their age. In this single-center cohort of 367 consecutive healthy and neurologically asymptomatic girls with precocious puberty, none had newly diagnosed pathological brain lesions. The only CPP-related incidental findings were Rathke's cleft cyst and pineal cyst.
A possible association between CPP and Rathke's cleft cyst has been reported in previous studies
1213; however, this condition rarely affects children and adolescents, and the incidence of pediatric Rathke's cleft cyst is unknown.
14 Jung et al.
15 and Lim and Yang
12 reported that 36 of 91 patients (39.5%) and 8 of 44 patients (18.1%) with Rathke's cleft cyst also had CPP; however, we identified Rathke's cleft cyst in only 7 of the 367 patients with CPP (1.9%). Our patients did not have any clinical symptoms of Rathke's cleft cyst and did not require treatment. Pineal cysts commonly occur in pediatric populations, with a higher prevalence observed among girls and older patients.
16 These cysts can occasionally cause precocious puberty among girls. We detected cases of suspected pituitary gland hyperplasia, which is a frequent cause of incidental findings and may cause precocious puberty; however, the incidence of pituitary hyperplasia in the general population is unknown.
17 In the present study, pituitary hyperplasia was considered to be a bulging contour of the pituitary gland with a height greater than 6 mm.
18 Patients who underwent MRI at 6–12 months after their diagnosis and others who did not undergo MRI were followed-up clinically; no pituitary gland changes or clinical findings suggestive of pituitary adenoma were observed. Kornreich et al.
7 found that CPP causes the pituitary to be prematurely larger than the reference range for age. Therefore, the patients with suspected pituitary gland hyperplasia likely had normal glands. We also detected cases of suspected pituitary hypoplasia and microadenoma, although no studies have reported on these conditions in CPP. The cases of suspected pituitary hypoplasia and microadenoma did not exhibit clinical symptoms, and it is difficult to draw conclusions regarding the relevance of these findings. Nevertheless, all the MRI findings in the present study may apply to healthy individuals as well; therefore, it remains unclear if they were causally related to CPP.
Many studies have examined the need for brain MRI in patients with CPP, and have attempted to identify the predictors associated with pathological intracranial abnormalities.
410192021 For example, Ng et al.
10 evaluated 67 girls in whom the mean age at the reported time of CPP onset was 6.2 years (range 2.0–7.9) and who were diagnosed with CPP using brain MRI; they concluded that cranial MRI scans were indicated for all girls with CPP because 15% of these girls had intracranial lesions that were not predicted by any of the examined factors (e.g., onset of puberty, pubertal stage, BA advancement, pelvic ultrasound findings, and GnRH stimulation test results). None of those patients had neurologic abnormalities at initial presentation. A study on 208 girls with CPP performed in Copenhagen (1993–2009)
4 revealed that 20 girls (9.6%) had incidental findings (e.g., pineal cyst or pituitary microadenoma), and 13 (6.3%) had pathological findings (e.g., arachnoid cyst, hypothalamic hematoma, and pilocytic astrocytoma). However, none of the patients had CNS signs or symptoms, and only one patient (0.5%) required surgery. Nevertheless, the researchers concluded that 6- to 8-year-old girls with CPP had a high frequency of intracranial lesions that were not associated with any useful predictors; in addition, they concluded that these girls should undergo brain MRI. In contrast, Bridges et al.
3 reported that of 96 girls with CPP, CNS abnormalities were found in only one patient with known brain lesions, and they concluded that an indication for brain MRI could not be justified based on the improved management of children with CPP alone. In addition, Pedicelli et al.
5 evaluated 182 girls with CPP during 1990–2012, and reported that 157 patients (86%) had normal findings, 19 (11%) had incidental findings, and 6 (3%) had pathological CNS abnormalities (e.g., hamartoma) that were detected before age 6 years and did not require surgery. None of the patients had signs or symptoms of CNS lesions. Therefore, Pedicelli et al.
5 concluded that intracranial pathologies occur rarely among girls with CPP older than 6 years.
The present study revealed results similar to those of previous studies. This may be related to the increasing incidence of CPP reported in the United States, Europe, China, and Korea.
222324 Furthermore, the annual incidence of CPP has substantially increased among Korean girls over the past 7 years, and the incidence among girls aged 6 years or older is significantly higher than that among girls younger than 6 years.
23 Based on the results of the present study, this finding was most likely caused by an increase in the idiopathic form of CPP rather than an increase in the frequency of brain lesions.
Some researchers believe that routine brain MRI is unnecessary for 6- to 8-year-old girls with CPP, and our findings support this view. Our center does not routinely perform sellar MRI for all children older than 6 years who are diagnosed with CPP. The researchers at our center explained the relationship between CPP and brain lesions to the parents of girls with CPP. Then, they performed sellar MRI only if the parents wanted it. Therefore, only 317 of 3,528 patients who were 6–8 years old underwent MRI. The remaining patients did not undergo sellar MRI and were diagnosed with suspected idiopathic CPP and treated with a GnRH agonist. They consistently underwent physical examinations for neurological symptoms. The 3,200 girls with suspected idiopathic CPP who did not undergo MRI had a mean follow-up period of 28.51 ± 17.09 months (range, 1–131 months), and none of these patients developed signs of intracranial lesions during the study period.
There is a consensus that girls who develop CPP before the age of 6 years may have a CNS lesion, and CPP in these cases is likely caused by a brain lesion. Therefore, we typically perform MRI for children who develop CPP before age 6 years. However, we found that none of the girls younger than 6 years with newly diagnosed CPP had a pathological brain lesion, with the exception of one 6-year-old girl with CPP who had a choroid plexus papilloma that was diagnosed at age 6 months. Despite its single-center design, this study identified 55 patients who were younger than 6 years and had CPP, which is comparable to the sample sizes of previous studies. Although we cannot definitively calculate the prevalence of CPP with concurrent intracranial lesions among children younger than 6 years, we believe that it is important to note the discrepancy between our findings and those of previous studies. This discrepancy does not change the need to screen CPP patients younger than 6 years for brain lesions, even with the absence of useful predictive factors; however, it is important to consider if the prevalence of CPP with concurrent brain lesions is overestimated.
Although CPP can be related to brain pathologies, relatively few cases require surgical intervention, and there is a relatively low risk associated with minor abnormalities and incidental findings. It may be unnecessary to routinely perform MRI to detect non-interventional conditions, especially because it is expensive and places an economic burden on patients. Therefore, the low frequency of the detection of interventional conditions may indicate that sellar MRI is not cost-effective. The non-routine use of MRI for patients with CPP was associated with savings of approximately USD 2,230,000 (USD 700/person during 2003–2016). Furthermore, no clinical findings were suggestive of brain lesions in patients who did not undergo sellar MRI, which indirectly confirms that there were no intracranial lesions that required intervention. Therefore, we believe that it may be appropriate to educate the parents of patients with CPP regarding the low probability of their children having dangerous brain pathologies. These parents could be guided away from routine MRI and toward simpler monitoring methods, such as continuous monitoring for neurological symptoms and altered growth status.
910
The present study had several limitations. First, it evaluated the patients' ages at the time of the diagnosis, and the precise onset of secondary sexual characteristic development was unknown because the parents did not report the age at onset. Therefore, the onset of secondary sexual characteristic development would have occurred at ages younger than those we identified. In addition, although the patients' mothers did precisely report the age at first menstruation, the maternal age of menarche could not be identified. Second, a single-center design is associated with known risks of bias, although we believe that the large sample size and prolonged study period support the validity of our findings. Third, the prevalence of brain lesions among patients who did not undergo sellar MRI remains unclear, although the long-term follow-up results indicated that there were no intracranial lesions that required intervention. However, the results of 3,200 patients who have not undergone sellar MRI are not negligible considering the study population, but this limitation did not affect the outcomes of the 317 patients who underwent sellar MRI. Therefore, these results support our belief that routine brain MRI may be unnecessary for 6- to 8-year-old girls with CPP. Finally, we examined only Korean children. Therefore, it is possible that the regional prevalence of brain lesions in CPP cases is related to the studied population, race, medical system, and social factors (e.g., access to medical care and resources for diagnosing CPP). As no new trends in the prevalence associated with brain lesions and CPP have been reported in the past decade, re-evaluations should be performed in other countries.
In conclusion, the present study revealed that the previously reported prevalence of cerebral lesions among girls with CPP was much higher than the prevalence observed at our center. Although it is possible that there are regional or racial differences in the prevalence of CPP, we believe that the prevalence of pathological cerebral lesions among CPP cases is overestimated and should be re-evaluated. Furthermore, it may be prudent to reconsider the routine use of brain MRI to screen all patients with CPP, especially if they are healthy and neurologically asymptomatic, and in the case of girls aged 6–8 years.







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download