Abstract
The authors present a rare of prenatally diagnosed congenital anaplastic astrocytoma. A 9-month-old boy had three recurrences despite two surgical resections and various chemotherapeutic regimens. He underwent the 3rd gross tumor removal at 11 months of age, followed by proton therapy, and now he remains disease-free for 3 yr without a significant neurocognitive dysfunction. This is the 1st case of a pediatric tumor treated by proton therapy in Korea, and proton therapy may be a treatment of choice for a congenital anaplastic astrocytoma in infants and young children, considering limitation of radiation therapy.
Congenital brain tumors are rare comprising only 0.5% to 1.5% of all pediatric brain tumors (1, 2), and are predominantly located in the supratentorial area. Furthermore, only 0.043% of all intracranial neoplasms are diagnosed in the prenatal period (3, 4). In congenital brain tumors, astrocytomas are the most common histological types (9.5%-28.9%) (4, 5).
The prognosis of infants and very young children with congenital astrocytomas, especially in the form of anaplastic histology is extremely poor. Because of the infiltrative nature in these anaplastic tumors, complete surgical resection is rarely accomplished, and because of the adverse effect of radiation therapy in this young age group, cranial radiation is also prohibitive. Furthermore, administration of multiagent adjuvant chemotherapy especially without the benefit of preceding gross surgical resection fails to improve the outcome.
We report here an infant with a congenital infratentorial anaplastic astrocytoma who is successfully treated with proton therapy following multiple surgical tumor resections and chemotherapy.
In January 2007, a 9-month-old boy with recurrent anaplastic astrocytoma of occipital lobe was transferred to our hospital for further management. On referral, he looked chronically ill but was adequate development for his age. Neurologically, he showed grade IV right sided weakness. He was born at 40 weeks of gestational age by normal vaginal delivery and the baby weighed 3.1 kg at birth. An obstetric ultrasound examination showed a huge mass in occipital lobe (64×46 mm) at the gestational age of 38 weeks. At birth, he had no other physical abnormalities (head circumference 34 cm, 50-75 percentile). A brain magnetic resonance imaging (MRI) (Fig. 1) at 1 week of age revealed a huge lobulated, soft tissue mass on parietooccipital lobe. Subtotal resection of the mass done at 2 weeks after birth confirmed anaplastic astrocytoma (Fig. 2). At 1 month of age, he was treated with chemotherapy as per the Korean Society for Pediatric Neuro-Oncology (KSPNO) infant brain tumor protocol (vincristine, etoposide, cisplatin, cyclophosphamide, carboplatin, ifosfamide) for 4 months until a follow-up brain computed tomography (CT) showed progressive tumor of 35×34 mm-sized mass in the occipital lobe. At 5 months of age, he underwent 2nd tumor resection. The tumor histology was the same as before. Within 2 weeks after the surgery, he was placed on temozolomide with no avail. At 7 months of age, chemotherapy was changed to irinotecan, celecoxib, etoposide and thalidomide. At 9 months of age, upon showing further progression of tumor on brain MRI, he was finally referred for further management.
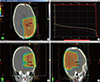
Upon arrival, he underwent the 3rd gross tumor removal at the age of 11 months and had post-operative recovery for two months. He was able to walk holding with right side weakness (grade IV), make eye contact and have control over his head at that time. And treatment strategy was followed by proton therapy to tumor bed for a total dose 40 cobalt gray equivalent (CGE)/20 fractions (Fx) in 4 weeks (Fig. 3). At 20 months of age, he was able to walk without assistance. Neurologically, he continued to have limping gait on the right. Neurocognitive function test by Korean-Wechsler Preschool and Primary Scale of Intelligence (K-WPPSI) at 38 months of age showed verbal intelligence 109, performance intelligence 71 and total intelligence 88 and he showed adequate development and at 51 months of age showed verbal intelligence 133, performance intelligence 108 and total intelligence 125. Now at 53 months of age, he remains disease-free with adequate head size for his age and the height of 50 percentile. The most recent follow up of brain MRI (3 yr post-proton therapy) showed a large surgical defect at left parietooccipital operative bed without evidence of tumor recurrence (Fig. 4).
In children, anaplastic astrocytoma, glioblastoma multiforme, and mixed glial tumors with a preponderance of malignant astrocytic elements collectively compose the family of malignant or high-grade astrocytic tumors. The majority of these tumors representing 7% to 11% of childhood central nervous system (CNS) tumors occur in the cerebral hemispheres (6-9). The median age at diagnosis is 9 to 10 yr, and the male-to-female ratio is near unity (7, 9).
Standard treatment of high-grade gliomas usually consists of cytoreductive surgery followed by radiation therapy and possibly chemotherapy. The extent of surgical resection regardless of the primary tumor site is the most important clinical prognostic factor (6-10).
In general, treatment of recurrent high-grade astrocytoma requires repeated surgical resections, focal irradiation, and perhaps systemic therapy as well. The same philosophy that pertains to aggressive resection at the time of initial presentation should also apply at the time of tumor progression.
Because of the fact that most lesions recur at the primary site, malignant gliomas often are amenable to reoperation at the time of disease progression. Although the majority of children succumb to further disease progression despite additional intervention, attempted re-resection is warranted in tumors that are amenable to gross total or radical subtotal removal (11).
Radiation therapy, as primarily a localized form of treatment, strives for improved local tumor control and cure with few adverse effects. Despite major advances in radiation therapy, side effects persist in a substantial proportion of patients, especially those who are vulnerable because of their age, tumor location, and a number of treatment-related factors, notably surgery (12-14). Brain development is most rapid during the first 3 yr of life. Development of white matter alterations after irradiation appears to mediate the functional and neurocognitive changes most concerning with regard to functional integrity (15). So radiation therapy should be delayed for young patients and identifies the potential benefits of reducing radiation dose to normal brain (16).
Proton therapy has been in clinical use since the 1970's and it is useful in treating a variety of cancers that include intracranial tumors, arteriovenous malformations (AVMs), sarcomas, head & neck cancers, tumors of the eye and orbit - such as ocular melanomas, retinoblastomas, prostate cancer, spine tumors (chordomas, chondrosarcomas), thoracic cancers and gastrointestinal cancers (unresectable liver tumors). At present, there is increasing interest in the proton therapy for pediatric tumors especially when a reduction in normal tissue radiation dose is of particular importance. There have been a number of publications comparing proton and photon dose distributions for pediatric patients with solid tumors (17-20). All show specific advantages for protons in reducing dose to normal tissues.
In our case of a rare congenital anaplastic astrocytoma as described here, he was treated with various chemotherapy regimens for several months after surgery. Despite of these treatments, the tumor recurred three times, he finally underwent the 3rd gross tumor removal at the age of 11 months. Considering his age and neurocognitive changes after conventional radiotherapy, proton therapy was used instead of conventional radiotherapy. Proton therapy to tumor bed for a total dose 40 CGE/20 Fx in 4 weeks was performed, in two months after the 3rd gross tumor removal. Only with the repeated surgical resections followed by proton therapy, patient remained disease-free for 3 yr without causing significant developmental problems and neurocognitive changes. Rather, the result of neurocognitive function test has been improved compared to previous study with continuous rehabilitation therapy. Before 2007, there was no proton therapy center in Korea. Proton therapy was started at proton therapy center of National Cancer Center in 2007. Since then, patients with variety of pediatric cancers have been treated by proton therapy. This is the 1st case of a pediatric brain tumor treated by proton therapy in Korea, without significant neurocognitive changes. This study, therefore, illustrates that proton therapy preceded by gross tumor removal even in the case of repeated recurrence may be a treatment of choice for a congenital anaplastic astrocytoma in infants and young children, considering limitations of radiation therapy.
Figures and Tables
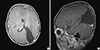
Fig. 1
Brain MRI at 1 week after birth showing a well-defined, purely solid mass occupying entire left occipital lobe, measuring about 70×42×50 mm. The mass shows homogeneous isointensity with gray matter on all sequences and subtle enhancement in the inferior aspect of the tumor mass. (A) Axial, T1 weighted image, (B) Axial, T2 weighted image, (C) Sagittal, T1 weighted image, (D) Sagittal, T2 weighted image.

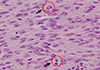
Fig. 2
Photomicrograph showing diffuse proliferation of astrocytes with large nuclei and frequent mitoses (arrows) obtained in a biopsy from the initial surgery (H&E, original magnification×400).
Notes
References
1. Balestrini MR, Micheli R, Giordano L, Lasio G, Giombini S. Brain tumors with symptomatic onset in the first two years of life. Childs Nerv Syst. 1994; 10:104–110.
2. Jooma R, Kendall BE. Intracranial tumours in the first year of life. Neuroradiology. 1982; 23:267–274.
3. Chung SN, Rosemond RL, Graham D. Prenatal diagnosis of a fetal intracranial tumor. J Ultrasound Med. 1998; 17:521–523.
4. Oi S, Kokunai T, Matsumoto S. Congenital brain tumors in Japan (ISPN Cooperative Study): specific clinical features in neonates. Childs Nerv Syst. 1990; 6:86–91.
5. Buetow PC, Smirniotopoulos JG, Done S. Congenital brain tumors: a review of 45 cases. AJR Am J Roentgenol. 1990; 155:587–593.
6. Campbell JW, Pollack IF, Martinez AJ, Shultz B. High-grade astrocytomas in children: radiologically complete resection is associated with an excellent long-term prognosis. Neurosurgery. 1996; 38:258–264.
7. Finlay JL, Boyett JM, Yates AJ, Wisoff JH, Milstein JM, Geyer JR, Bertolone SJ, McGuire P, Cherlow JM, Tefft M, et al. Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen: Childrens Cancer Group. J Clin Oncol. 1995; 13:112–123.
8. Heideman RL, Kuttesch J Jr, Gajjar AJ, Walter AW, Jenkins JJ, Li Y, Sanford RA, Kun LE. Supratentorial malignant gliomas in childhood: a single institution perspective. Cancer. 1997; 80:497–504.
9. Sposto R, Ertel IJ, Jenkin RD, Boesel CP, Venes JL, Ortega JA, Evans AE, Wara W, Hammond D. The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: results of a randomized trial: a report from the Childrens Cancer Study Group. J Neurooncol. 1989; 7:165–177.
10. Wisoff JH, Boyett JM, Berger MS, Brant C, Li H, Yates AJ, McGuire-Cullen P, Turski PA, Sutton LN, Allen JC, et al. Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: a report of the Children's Cancer Group trial no. CCG-945. J Neurosurg. 1998; 89:52–59.
11. Finlay JL, Goldman S, Wong MC, Cairo M, Garvin J, August C, Cohen BH, Stanley P, Zimmerman RA, Bostrom B, et al. Pilot study of high-dose thiotepa and etoposide with autologous bone marrow rescue in children and young adults with recurrent CNS tumors: the Children's Cancer Group. J Clin Oncol. 1996; 14:2495–2503.
12. Reimers TS, Ehrenfels S, Mortensen EL, Schmiegelow M, Sønderkaer S, Carstensen H, Schmiegelow K, Müller J. Cognitive deficits in long-term survivors of childhood brain tumors: identification of predictive factors. Med Pediatr Oncol. 2003; 40:26–34.
13. Robertson PL, Muraszko KM, Holmes EJ, Sposto R, Packer RJ, Gajjar A, Dias MS, Allen JC. Children's Oncology Group. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children's Oncology Group. J Neurosurg. 2006; 105:444–451.
14. Sønderkaer S, Schmiegelow M, Carstensen H, Nielsen LB, Müller J, Schmiegelow K. Long-term neurological outcome of childhood brain tumors treated by surgery only. J Clin Oncol. 2003; 21:1347–1351.
15. Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973; 48:757–767.
16. Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009; 27:3691–3697.
17. Fuss M, Hug EB, Schaefer RA, Nevinny-Stickel M, Miller DW, Slater JM, Slater JD. Proton radiation therapy (PRT) for pediatric optic pathway gliomas: comparison with 3D planned conventional photons and a standard photon technique. Int J Radiat Oncol Biol Phys. 1999; 45:1117–1126.
18. Lee CT, Bilton SD, Famiglietti RM, Riley BA, Mahajan A, Chang EL, Maor MH, Woo SY, Cox JD, Smith AR. Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: how do protons compare with other conformal techniques? Int J Radiat Oncol Biol Phys. 2005; 63:362–372.
19. Mu X, Björk-Eriksson T, Nill S, Oelfke U, Johansson KA, Gagliardi G, Johansson L, Karlsson M, Zackrisson DB. Does electron and proton therapy reduce the risk of radiation induced cancer after spinal irradiation for childhood medulloblastoma? a comparative treatment planning study. Acta Oncol. 2005; 44:554–562.
20. Weber DC, Trofimov AV, Delaney TF, Bortfeld T. A treatment planning comparison of intensity modulated photon and proton therapy for paraspinal sarcomas. Int J Radiat Oncol Biol Phys. 2004; 58:1596–1606.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download