Abstract
Waldenstrom macroglobulinemia (WM) is a B-cell lymphoproliferative disorder associated with bone marrow involvement of lymphoplasmacytic lymphoma (LPL) and an IgM monoclonal gammopathy. Generally B-lymphocytes in LPL do not express CD5 that is important for differential diagnosis of B-cell lymphoproliferative disorders. In WM, various renal diseases and type I cryoglobulinemia are well described separately, but cryoglobulinemic glomerulonephropathy is very rarely reported. A 61-yr-old woman complained of generalized edema, cyanosis of the extremities in cold weather, visual disturbance, and pancytopenia. Bone marrow and renal biopsy showed CD5+ expressing B-cells and cryoglobulinemic glomerulonephropathy. With the diagnosis of WM, she received cyclophosphamide, doxorubicin, vincristine and prednisolone chemotherapy and got complete remission. Here, we report a rare case of WM associated with unusual expression of CD5+ B-lymphocytes and cryoglobulinemic glomerulonephropathy, and emphasize the importance of the clinical features in differentiating CD5+ B-cell lymphoproliferative disorders.
Waldenstrom macroglobulinemia (WM) is a rare plasma cell disorder characterized by lymphoplasmacytic infiltration of bone marrow and by the demonstration of an IgM paraproteinemia (1). By the definition of the World Health Organization (WHO) and the Revised European-American Lymphoma (REAL) classification criteria, WM is classified as an indolent form of B-cell non-Hodgkin's lymphoma known as lymphoplasmacytic lymphoma (LPL) (2). LPL is a neoplasm of small B lymphocytes, plasmacytoid lymphocytes, and plasma cells, with variable numbers of immunoblasts (3). In LPL, small B lymphocytes are usually negative for CD5, CD10, and CD23, so LPL is presumed to derive from CD5 negative peripheral B lymphocytes stimulated to differentiate to plasma cells (4). In WM, only approximately 5%-20% of cases had CD5 positivity (5). The incidence of WM is not recognized in Asia, and LPL constitutes less than 5% of all non-Hodgkin lymphomas and is extremely rare in patients of East Asia (4). So the incidence of WM of CD5 positive LPL in Asia is guessed to be very uncommon.
On the other hand, clinical features of WM are associated with tissue infiltration by neoplastic cells and IgM monoclonality (6). In WM, renal disease and type I cryoglobulinemia are well recognized, separately, but cryoglobulinemic glomerulonephropathy is very rare and only 2 cases have been reported worldwide (7, 8).
Now we report a rare case of WM associated with CD5+ LPL and presented as cryoglobulinemic glomerulonephropathy.
On April 24, 2009, a 61-yr-old woman visited our hospital with generalized edema for 3 months. She also complained of tingling sensation and cyanosis of extremities in cold weather and visual disturbance. Physical examination showed mild anemic conjunctivae, splenomegaly and pretibial pitting edema on both legs. But there were no superficial lymph nodes and hepatomegaly. The laboratory findings revealed white blood cells, 3,270/µL (segment neutrophil 57%; lymphocytes 28%); hemoglobin, 8.8 g/dL; and platelets, 81,000/µL. Blood urea nitrogen, serum creatinine, total protein and albumin were 28.2 mg/dL, 1.4 mg/dL, 5.7 g/dL and 3.0 g/dL, respectively. In urinalysis, 4+ of proteinuria and 3+ of hematuria were found. Bence-Jones proteins were not found in urine. ANA was positive (1:640, speckled) and anti-ds DNA antibody was negative. The serum levels of IgG, IgA were normal (IgG, 972.47 mg/dL [normal 970-1,700 mg/dL], IgA, 242.07 mg/dL [normal 110-410 mg/dL]) but IgM was slightly elevated (246.41 mg/dL [normal 35-220 mg/dL]). C3 was 55.45 mg/dL (normal 86-160 mg/dL) and C4 was 1.6 mg/dL (normal 17-45 mg/dL). Serum LDH was 300 IU/L. HBsAg, anti-HBs and anti-HCV were all negative. In renal biopsy, we found that the glomeruli are generally enlarged showing marked endocapillary proliferation and irregularly thickened capillary wall with frequent obliteration of the capillary lumen due to pseudothrombi and subendothelial hyaline deposits with stained IgM. On the electron microscopic (EM) findings, there are subendothelial and mesangial electron dense deposits revealing microtubular structures compatible with cryoglobulinemic glomerulonephropathy (Fig. 1). In additional tests, cryoglobulin was positive and the level of β2-microglobulin was 4.4 mg/L (normal 0-2.4 mg/L). Monoclonal gammopathy with IgM kappa type was seen in immunofixation electrophoresis (Fig. 2A). Bone marrow biopsy revealed small lymphocytes with expression of CD5+CD20+ suggestive of B-cell lymphoproliferative disorders and positive reaction in small numbers of plasma cells with anti-cytoplasmic IgM antibody (Fig. 3). Ophthalamic examination showed vascular segmentation and dilatation of the retinal veins (Fig. 4) and PET-CT showed no abnormal hypermetabolic lesions. Thus, on the basis of symptoms, signs, the laboratory findings, renal biopsy and bone marrow examination, we diagnosed WM. She was treated with cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) chemotherapy. After the treatment, we observed negative proteinuria, hematuria, serum cryoglobulin and monoclonal gammopathy in immunofixation electrophoresis (Fig. 2B). Complete blood cell profiles were normalized and lymphoid cell aggregation in bone marrow was absent. And she also had improved visual acuity and tingling sensation of upper and lower extremities.
According to the criteria of Waldenstrom Macroglobulinemia (WM) revised in the 4th edition of the WHO classification in 2008, the definition of WM combines the morphological diagnosis of bone marrow involvement of lymphoplasmacytic lymphoma (LPL) with IgM paraproteinemia (9). Owing to histological overlapping between lymphoplasmacytic lymphoma and other mature B-cell malignancies with plasmacytic differentiation, immunophenotyping is invaluable in the differential diagnosis of B-cell lymphoproliferative disorders, and in all cases of suspected WM it is needed to apply (5). The special immunophenotypic profile for lymphoplasmacytic cells in WM include the expression of monoclonal immunoglobulin light chain (κ and λ and the pan B-cell antigens CD19+CD20+ (1). The majority of cases have CD5-CD10-CD23-, but 5%-20% of patients appear to express the CD5 antigen (10). Although the expression of CD5+ in LPL/WM is uncommon, this should not preclude a diagnosis of WM. However, the attention should be paid to diagnose the CD5+ cases to exclude chronic lymphocytic leukemia and mantle cell lymphoma.
In bone marrow examination of our patient, small lymphoid cells expressed CD5+CD20+ and positive reaction in small numbers of plasma cells with anti-cytoplasmic IgM antibody was observed. Because of CD5+ small lymphoid cells, the careful attention for symptoms and signs was important to differentiate from other B-cell lymphoproliferative disorders. Comparing to chronic lymphocytic leukemia, peripheral blood lymphocyte count of this patient was not compatible with the diagnostic criteria of the International Workshop on CLL (IW-CLL) (11). In mantle cell lymphoma, swelling of lymph nodes are usually present and gastrointestinal tract involvement occurs in a very high percentage and the majority of patients have genetic abnormalities such as t(11:14)(q13;q32) (3, 12). This patient did not have lymphadenopathy and extranodal involvement in PET-CT and also did not have chromosome abnormalities. Instead of these characteristics of other B-cell lymphoproliferative disorders, this patient complained of hematuria, pretibial pitting edema, numbness of hand and foot in cold weather, and visual disturbance (13).
Renal involvement of WM is a rare manifestation, but a wide spectrum of renal lesions is observed. After the first description of human renal lesions associated with deposition of monoclonal lights chains, several renal complications have been reported (7). WM-related nephropathies are characteristic intracapillary deposits of IgM. In renal biopsy of our patient, we found pseudothrombi and subendothelial hyaline deposits with stained IgM and subendothelial and mesangial electron dense deposits revealing microtubular structures on electron microscopy compatible with cryoglobulinemic nephropathy (14). Serum cryoglobulin of this patient was positive. Although there are a number of systemic conditions associated with cryoglobulinemia, including hepatitis C, lymphoproliferative disorders, infections, systemic lupus erythematosus (SLE) and other collagen vascular diseases, this patient was not associated with them. In WM, cryoglobulinemic glomerulonephropathy is a very rare renal disease which was reported in only 2 cases worldwide (7, 8).
In addition to renal disease, the patient complained of visual disturbance. Ophthalamic examination showed vascular segmentation and dilatation of the retinal veins. These revealed hyperviscosity syndrome (6). Hyperviscosity is a main feature in WM patients, and is due to the excess of IgM paraproteins. The relationship between serum viscosity and clinical manifestations is not precise, and a general absolute value for viscosity at which hyperviscosity becomes clinically relevant does not exist (15). And it is difficult to use commercial check of viscosity in Korea, so we couldn't run the tape over serum viscosity level.
WM usually follows a relatively indolent course with a median survival ranging from 60 to 120 months in different series (16). However, in some patients the disease may be more aggressive (16). Several analyses have identified variables that may be associated with a worse prognosis. These include advanced age, cytopenia(s), hypoalbuminemia, elevated serum β2-microglobulin, high IgM, poor performance status, B-symptoms or splenomegaly. An International Prognositc Scoring System for WM (IPSSWM) was formulated based on 5 adverse covariates: age > 65 yr, hemoglobin less than or equal to 11.5 g/dL, platelet count less than or equal to 100,000/µL, β2-microglobulin more than 3 mg/L, and serum monoclonal protein concentration more than 7.0 g/dL. The risk group is categorized as having low, intermediate, high risk and five-year survival rates of these groups are 87%, 68%, and 36% respectively (17). Meanwhile, the indications for immediate therapy are disease-related cytopenias, bulky adenopathy or organomegaly, symptomatic hyperviscosity, severe neuropathy, amyloidosis, cryoglobulinemia, cold agglutinin disease, or evidence of disease transformation (13). There is no standard therapy for the treatment of symptomatic WM. Recent studies recommended the combination of rituximab with nucleoside analogs and/or alkylating agents or with cyclophosphamide-based therapies, or the combination of rituximab with thalidomide (13). An alternative frontline regimen can be considered along with bortezomib, alemtuzumab and stem cell transplantation. Newer agents, such as bendamustine and everolimus, can also be considered in the treatment of WM (6). According to IPSSWM, our patient is high risk group based on hemoglobin, platelet count and β2-microglobulin level. However, at present, no data suggest that new targeted therapies may overcome an adverse prognostic factor in WM and rituximab-based chemotherapy has economic problem in Korea. So we treated this patient with CHOP chemotherapy and after the treatment, the patient was in complete remission.
In conclusion, WM associated with CD5+ LPL and presented as cryoglobulinemic glomerulonephropathy is very rare, so clinical features are important for differential diagnosis in CD5+ B-cell lymphoproliferative disorders. Conventional chemotherapy with CHOP is effective in symptomatic high risk group with cryoglobulinemia in WM.
Figures and Tables
Fig. 1
Renal biopsy. (A) Immunofluorescent microscopic study showed 2+ reaction for IgM. (B) On the electron microscopic (EM) findings (× 20,000), there are subendothelial (arrow) and mesangial electron dense deposits revealing microtubular structures (25 nm in average diameter).
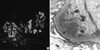
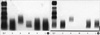
Fig. 2
Serum immunofixation electrophoresis. (A) There is a slightly dense band with IgM, kappa antisera, suggestive of monoclonal gammopathy (B) After the treatment, a dense band with IgM was disappeared.
References
1. Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML, Morra E, Pangalis GA, San Miguel JF, Branagan AR, Dimopoulos MA. Clinicopathological definition of Waldenstroms macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003. 30:110–115.
2. Stone MJ, Pascual V. Pathophysiology of Waldenström's macroglobulinemia. Haematologica. 2010. 95:359–364.
3. Fisher RI, Mauch PM, Harris NL, Friedberg JW. DeVita VT, Hellman S, Rosenberg SA, editors. Non-Hodgkin's lymphoma. Cancer: Principles and practice of oncology. 2005. 7th ed. Philadephia, PA: Lippincott Williams & Wilkins;1957–1997.
4. Won YW, Kim SJ, Kim K, Ko YH, Kim WS. Clinical features and treatment outcomes of lymphoplasmacytic lymphoma: a single center experience in Korea. Ann Hematol. 2010. 89:1011–1018.
5. Morice WG, Chen D, Kurtin PJ, Hanson CA, McPhail ED. Novel immunophenotypic features of marrow lymphoplasmacytic lymphoma and correlation with Waldenström's macroglobulinemia. Mod Pathol. 2009. 22:807–816.
6. Treon SP. How I treat Waldenström macroglobulinemia. Blood. 2009. 114:2375–2385.
7. Audard V, Georges B, Vanhille P, Toly C, Deroure B, Fakhouri F, Cuvelier R, Belenfant X, Surin B, Aucouturier P, Mougenot B, Ronco P. Renal lesions associated with IgM-secreting monoclonal proliferations: revisiting the disease spectrum. Clin J Am Soc Nephrol. 2008. 3:1339–1349.
8. Tomiyoshi Y, Sakemi T, Yoshikawa Y, Shimokama T, Watanabe T. Fibrillar crystal structure in essential monoclonal IgM kappa cryoglobulinemia. Clin Nephrol. 1998. 49:325–327.
9. Ott G, Balague-Ponz O, de Leval L, de Jong D, Hasserjian RP, Elenitoba-Johnson KS. Commentary on the WHO classification of tumors of lymphoid tissues (2008): indolent B cell lymphomas. J Hematop. 2009. 2:77–81.
10. Konoplev S, Medeiros LJ, Bueso-Ramos CE, Jorgensen JL, Lin P. Immunophenotypic profile of lymphoplasmacytic lymphoma/Waldenström macroglobulinemia. Am J Clin Pathol. 2005. 124:414–420.
11. Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, Kipps TJ. International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008. 111:5446–5456.
12. Ghielmini M, Zucca E. How I treat mantle cell lymphoma. Blood. 2009. 114:1469–1476.
13. Dimopoulos MA, Gertz MA, Kastritis E, Garcia-Sanz R, Kimby EK, LeBlond V, Fermand JP, Merlini G, Morel P, Morra E, Ocio EM, Owen R, Ghobrial IM, Seymour J, Kyle RA, Treon SP. Update on treatment recommendations from the Fourth International Workshop on Waldenstrom's Macroglobulinemia. J Clin Oncol. 2009. 27:120–126.
14. Herrera GA, Turbat-Herrera EA. Renal diseases with organized deposits: an algorithmic approach to classification and clinicopathologic diagnosis. Arch Pathol Lab Med. 2010. 134:512–531.
15. Vitolo U, Ferreri AJ, Montoto S. Lymphoplasmacytic lymphoma-Waldenstrom's macroglobulinemia. Crit Rev Oncol Hematol. 2008. 67:172–185.
16. Dimopoulos MA, Kastritis E, Delimpassi S, Zomas A, Kyrtsonis MC, Zervas K. The International Prognostic Scoring System for Waldenstrom's macroglobulinemia is applicable in patients treated with rituximab-based regimens. Haematologica. 2008. 93:1420–1422.
17. Morel P, Duhamel A, Gobbi P, Dimopoulos MA, Dhodapkar MV, McCoy J, Crowley J, Ocio EM, Garcia-Sanz R, Treon SP, Leblond V, Kyle RA, Barlogie B, Merlini G. International prognostic scoring system for Waldenstrom macroglobulinemia. Blood. 2009. 113:4163–4170.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download