Abstract
The aim of this study was to examine the relationship of complications related to diverticulitis and visceral obesity. The study was based on a retrospective case note review conducted at the Hanyang University Hospital. Patients were diagnosed with diverticulitis based on clinical symptoms and abdominal computed tomography (CT) findings and divided into two groups: those admitted with complicated diverticulitis and those with a simple diverticulitis episode. We compared the body mass index (BMI) and degree of visceral obesity, measured by abdominal CT. The study included 140 patients, 87 (62.1%) were simple diverticulitis and 53 (37.9%) were complicated diverticulitis. In the complicated diverticulitis group, 9 (6.4%) cases were recurrent, 29 (20.7%) were perforation or abscess patients, and 28 (20%) were patients with systemic inflammatory response syndrome (SIRS). Of the SIRS patients, 13 were involved in other complication groups. When comparing in the two groups, the complicated diverticulitis group had a significantly higher visceral fat area (128.57 cm2 vs 102.80 cm2, P = 0.032) and a higher ratio of visceral fat area/subcutaneous fat area (0.997 vs 0.799, P = 0.014). Visceral obesity is significantly associated with complications of diverticulitis.
Diverticular disease is relatively common. Colonic diverticulosis is etiologically related to a chronically low-fiber diet, and is more frequent in industrized countries (1). Diverticular disease is rare in people under the age of 40, and prevalence increases with age, although some studies have shown that the mean age of diverticular disease patients is decreasing (2). Although the prevalence of diverticular disease is increasing, most people with diverticular disease remain asymptomatic. Acute diverticulitis is the most common complication of colonic diverticulosis, with 10%-25% of diverticulosis patients developing diverticulitis (3, 4). Most patients are cured without complications, but 15% undergo severe complications such as abscess formation, bowel perforation, and recurrence (5).
Obesity has long been investigated as a cause of diseases such as type 2 diabetes mellitus, hypertension, osteoarthritis, gastrooesophageal reflux, obstructive sleep apnea and certain cancers (6). Recently, several studies have shown a relationship among obesity, diverticulitis and diverticulitis complications, with a positive correlation not only between obesity and prevalence of diverticulitis, but also between obesity and the rate of complications (1, 2). Usually, obesity refers to an excess of body fat, which can be divided into subcutaneous and visceral fat. The metabolic characteristics of these two kinds of fat are different, with visceral fat highlighted as a cause of metabolic complications such as hypertriglyceridemia, insulin resistance, and a proinflammatory condition (7, 8). We conducted this study to evaluate the relationship between diverticulitis complications and obesity, particularly visceral obesity.
The study was based on a retrospective case note review, conducted from 1 January 2003 to 31 December 2007 at Hanyang University Hospital in Seoul, Korea. We used the hospital database to find patients who met the International Code of Disease (ICD) for acute diverticulitis. From the initial patient list, we collected those diagnosed with diverticulitis from clinical symptoms and abdominal computed tomography (CT) findings. Patients with appendicitis misdiagnosed as diverticulitis, a history of bowel resection, or any gastrointestinal malignancy were excluded. We divided the patients into two groups: those admitted with complicated diverticulitis such as abscess formation, diverticular perforation, recurrent diverticulitis, and systemic inflammatory response syndrome, and those with a simple diverticulitis episode.
From medical records, we obtained patient height and weight at admission, and calculated the body mass index (BMI). Blood lipid profile (total cholesterol, triglyceride, low density lipoprotein, high density lipoprotein) was also obtained. To measure the amount of visceral and subcutaneous fat, we used the abdominal CT image data. After measuring the total and intraperitoneal area of the Haunsfield number -190 to -30 at the level of L3-4 vertebra, we used the intraperitoneal area as the visceral fat area (VFA) and the total area as the total fat area (TFA). Then we calculated the subcutaneous fat area (SFA) as the difference between TFA and VFA (Fig. 1).
To determine the relationship between diverticulitis complications and obesity, we compared the complicated and simple diverticulitis groups for BMI, total cholesterol, triglyceride, low density lipoprotein and high density lipoprotein. VFA, TFA, SFA, VFA/SFA, and VFA/TFA were compared between the groups to determine the correlation between visceral obesity and diverticulitis complications. An independent t-test was used for statistical analysis. A P value of less than 0.05 was considered to be statistically significant.
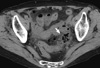
The medical records of 166 diverticulitis patients were reviewed, and 26 patients were excluded because 12 had acute appendicitis, 8 had a history of bowel resection, and 6 had colon cancer. A total of 140 patients were enrolled in this study, with 46 females (32.9%) and 94 males (67.1%) with a mean age of 43 ± 14 yr. The number of simple diverticulitis patients was 87 (62.1%) and the number of complicated diverticulitis patients was 53 (37.9%). The general characteristics of the two groups were not significantly different. In the simple diverticulitis group, 60.9% (53/87) were male, and in the complicated diverticulitis group 77.3% (42/53) were male (P = 0.063). The mean age of the simple diverticulitis group was 41.8 ± 14.9 and 44.8 ± 14.2 yr (P = 0.539) in the complicated diverticulitis group. Right sided colon lesions occurred in 94% (82/87) of simple diverticulitis cases and 91% (48/53) of complicated diverticulitis cases (P = 0.504) (Table 1). Fig. 2 shows a complicated case of diverticulitis. It shows a perforated sigmoid diverticulitis with surrounding air bubble.
In the complicated diverticulitis group, 9 patients (6.4%) were recurrent patients, 29 (20.7%) were perforation or abscess patients, and 28 (20%) were systemic inflammatory response syndrome (SIRS) patients. Of the SIRS patients, 13 were involved in other complication groups.
The mean BMI in the simple diverticulitis group was 23.3 kg/m2, and 24.1 kg/m2 in the complicated diverticulitis group. Although the BMI of the complicated diverticulitis group was higher than that of the simple diverticulitis group, the difference was not significant (P = 0.229). The TFAs of the complicated diverticulitis group and the simple diverticulitis group were 265.8 cm2 and 233.9 cm2, respectively, which was not significantly different (P = 0.119). SFA was also not significantly different between groups (137.2 cm2 vs 129.0 cm2, P = 0.463). When comparing VFA/SFA in the two groups, the complicated diverticulitis group was significantly higher (0.997 vs 0.799, P = 0.014), as it was for VFA/TFA (0.477 vs 0.418, P = 0.016). No significant difference was seen for total cholesterol (P = 0.598), HDL (P = 0.081) or LDL (P = 0.525). The complicated diverticulitis group had significantly higher VFA, VFA/SFA, and VFA/TFA, but no significant difference in TFA, BMI and blood lipid profile (Table 2). In multivariate analysis, VFA was only significant risk factor of complicated diverticulitis (Table 3).
Diverticulosis is a common disease, especially in western populations, with a suspected prevalence of 30%-35%. Diverticulosis is more frequent in the sigmoid colon, and is rare in the right colon in western countries (9). In Eastern areas such as Korea, Japan and Hong Kong, the prevalence is lower than in western countries, and the right colon is the most common site of diverticulosis. The prevalence of diverticulosis is increasing in Eastern countries due to the westernization of eating patterns (10). The prevalence of diverticular disease is also increasing in Korea (4, 11).
Although diverticular disease is common, only a small percentage of diverticular disease patients will develop diverticulitis, and few develop complicated diverticulitis. Many studies on the complications of diverticulitis have been published, but only a small number investigate the contributing factors (12). In 2005, Dobbins et al. (1) reported that obesity is a risk factor for diverticular complications such as perforation and recurrent episodes, based on the BMI difference between complicated and simple diverticulitis groups. Several other retrospective case series and a small case-controlled study noted a preponderance of obesity in diverticulitis patients (13-16). In all the studies, researchers compared a study group and a control group using only weight or BMI as a measure of obesity.
The mechanism by which obesity contributes to diverticulitis complications is not clear. However, obesity has been suggested to be a major cause of insulin resistance, hypertension, cardiovascular disease and a systemic inflammatory state (17, 18). Dusserre et al. (8) reported that visceral and subcutaneous adipocytes may have different properties regarding the production of bioactive molecules. In particular, visceral adipose tissue has a higher basic lipolysis rate and lower sensitivity to the inhibition of lipolysis by insulin (7). In addition to its metabolic activity, visceral adipose tissue produces large amounts of interleukin-6, a representative inflammatory cytokine. Adiponectin, a known anti-inflammatory material, has been documented to be lower in obese individuals. Of special interest, adiponectin plasma levels are more closely related to the amount of visceral fat rather than total fat (19). Therefore, expanded intra-abdominal fat deposits may contribute to a proinflammatory state, which in turn is linked to clinical events (20).
Based on these findings, we hypothesize that visceral obesity contribute to the development of complicated diverticulitis. In this study, in contrast to previous studies on obesity and diverticulitis complications, no significant difference in BMI (P = 0.229) was seen between complicated and simple diverticulitis. We deemed that these results are due to the fact that Asians tend to have higher prevalence of abdominal obesity, especially visceral fat accumulation, even with a lower body mass index (21). And we think our small study population can be another factor of these results. Instead, the VFA and the ratio of VFA/SFA were significantly different, suggesting that simple obesity is not the contributing factor, but that visceral obesity is a risk factor for complicated diverticulitis. We propose that this is caused by the different metabolic and inflammatory characteristics of visceral and subcutaneous adipose tissue, described above. Considering the effects of visceral obesity, accurate measurement of visceral fat is important, and measurement of adipose tissue area using CT images of the L4-5 vertebra level is estimated to be a useful method of visceral fat measurement (22). Thus, we believe that in this study, visceral and subcutaneous fat were properly measured.
Another important result of this study is the age of diverticulitis patients. Previous estimates of the percentage of diverticulitis patients younger than 40 yr old range from 2% to 5%, and acute diverticulitis has long been considered a disease that typically affects people over 50 (23, 24). Nonetheless, acute diverticulitis is evidently becoming more common in patients younger than 40, as previously suspected in western countries (14, 15, 25). The mean age of diverticulitis patients in this study population was 43, and the percentage of patients under age 40 was 46% (65/140). This mean age is much younger than that found in previous studies of the Korea population. Kim et al. (11) reported in 2003 that the mean age of acute diverticulitis was 53. Although the number of patients in our study was too small to represent all diverticulitis patients in Korea, our results reflect the increase in younger patients, similar to the pattern seen in western countries. A few studies have suggested that younger patients experience more severe disease courses with complications (26, 27). One factor that may contribute to this observation is the potential for a delay in acute diverticulitis diagnosis due to a low index of suspicion of diverticular disease in young adults (28). However, in our study, the mean ages for complicated and simple diverticulitis were 44.8 and 41.8 yr, with no significant difference between the groups. In our study, all patients were initially diagnosed by abdominal CT and physical examination, and abdominal CT is considered the most effective diagnostic method for diverticulitis (29). We propose that the increased frequency of abdominal CT examinations on a patient's first visit will increase the early diagnosis rate of diverticulitis and improve the clinical course of young diverticulitis patients.
Our study has several limitations. The study population was small, so the results do not represent the entire Korean population. Although we classified the complication of diverticulitis as recurrence, abscess formation/perforation and SIRS, some overlap occurred between SIRS and other complication groups. This was inevitable because SIRS is a systemic complication and the others are local complications. In this study, visceral obesity was associated with complicated diverticulitis, but we did not follow up with research to explain these results. We propose several hypotheses, including the different metabolic characteristics and cytokine excretion of visceral and subcutaneous fat, to drive further research.
In conclusion, the complicated diverticulitis group had a significantly higher visceral fat area and a higher ratio of visceral fat area/subcutaneous fat area. Visceral obesity is significantly associated with complications of diverticulitis.
Figures and Tables
Fig. 1
Measurement of total and visceral fat area of Haunsfield number -190~-30 at the level of L3-4 vertebra. Subcutaneous fat area is calculated by difference between total fat area and visceral fat area. (A) Measurement of total fat area; (B) Measurement of visceral fat area.

Fig. 2
Abdominal CT scan of complicated diverticulitis. The arrow shows a sigmoid colon wall infiltration with surrounding air bubbles.
AUTHOR SUMMARY
Correlation between Complicated Diverticulitis and Visceral Fat
Jong Heon Jeong, Hang Lak Lee, Jin Ok Kim, Hye Jin Tae, Suk Hyun Jung, Kang Nyeong Lee, Dae Won Jun, Oh Young Lee, Byung Chul Yoon, Ho Soon Choi, Joon Soo Hahm and Soon Young Song
Recently, several studies showed a correlation between diverticulitis with complication and obesity. Here we investigated a relationship between complications related to diverticulitis and 'visceral obesity'. 140 patients diagnosed with diverticulitis based on clinical symptoms and abdominal CT findings were divided into two groups: those with simple diverticulitis and those with complicated diverticulitis. Of all cases, 91 (65%) were simple diverticulitis and 49 (35%) were complicated diverticulitis. There were no significant difference in age, sex and location of lesion. However, the complicated diverticulitis group had a significantly higher visceral fat area (P = 0.032) and a higher ratio of visceral fat/subcutaneous fat (P = 0.014). We suggest that visceral obesity is significantly associated with complications of diverticulitis.
References
1. Dobbins C, Defontgalland D, Duthie G, Wattchow DA. The relationship of obesity to the complications of diverticular disease. Colorectal Dis. 2006. 8:37–40.
2. Zaidi E, Daly B. CT and clinical features of acute diverticulitis in an urban U.S. population: rising frequency in young, obese adults. AJR Am J Roentgenol. 2006. 187:689–694.
3. Kang JY, Melville D, Maxwell JD. Epidemiology and management of diverticular disease of the colon. Drugs Aging. 2004. 21:211–228.
4. Choi CS, Cho EY, Kweon JH, Lim PS, No HJ, Kim KH, Lee JG, Seo GS, Kim TH, Choi SC. The prevalence and clinical features of colonic diverticulosis diagnosed with colonoscopy. Korean J Gastrointest Endosc. 2007. 35:146–151.
5. Morris CR, Harvey IM, Stebbings WS, Speakman CT, Kennedy HJ, Hart AR. Epidemiology of perforated colonic diverticular disease. Postgrad Med J. 2002. 78:654–658.
6. Kral JG. Morbidity of severe obesity. Surg Clin North Am. 2001. 81:1039–1061.
7. Arner P. Differences in lipolysis between human subcutaneous and omental adipose tissues. Ann Med. 1995. 27:435–438.
8. Dusserre E, Moulin P, Vidal H. Differences in mRNA expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose tissues. Biochim Biophys Acta. 2000. 1500:88–96.
9. Painter NS, Burkitt DP. Diverticular disease of the colon: a deficiency disease of Western civilization. Br Med J. 1971. 2:450–454.
10. Miura S, Kodaira S, Aoki H, Hosoda Y. Bilateral type diverticular disease of the colon. Int J Colorectal Dis. 1996. 11:71–75.
11. Kim HU, Kim YH, Choe WH, Kim JH, Youk CM, Lee JU, Shim SG, Son HJ, Rhee PL, Kim JJ, Rhee JC. Clinical characteristics of colonic diverticulitis in Koreans. Korean J Gastroenterol. 2003. 42:363–368.
12. Mäkelä JT, Kiviniemi HO, Laitinen ST. Elective surgery for recurrent diverticulitis. Hepatogastroenterology. 2007. 54:1412–1416.
13. Konvolinka CW. Acute diverticulitis under age forty. Am J Surg. 1994. 167:562–565.
14. Mader TJ. Acute diverticulitis in young adults. J Emerg Med. 1994. 12:779–782.
15. Schauer PR, Ramos R, Ghiatas AA, Sirinek KR. Virulent diverticular disease in young obese men. Am J Surg. 1992. 164:443–446.
16. Schweitzer J, Casillas RA, Collins JC. Acute diverticulitis in the young adult is not "virulent". Am Surg. 2002. 68:1044–1047.
17. Larsson B, Svärdsudd K, Welin L, Wilhelmsen L, Björntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed). 1984. 288:1401–1404.
18. Calabro P, Yeh ET. Intra-abdominal adiposity, inflammation, and cardiovascular risk: new insight into global cardiometabolic risk. Curr Hypertens Rep. 2008. 10:32–38.
19. Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006. 444:881–887.
20. Mathieu P, Poirier P, Pibarot P, Lemieux I, Després JP. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009. 53:577–584.
21. McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991. 337:382–386.
22. Rössner S, Bo WJ, Hiltbrandt E, Hinson W, Karstaedt N, Santago P, Sobol WT, Crouse JR. Adipose tissue determinations in cadavers: a comparison between cross sectional planimetry and computed tomography. Int J Obes. 1990. 14:893–902.
23. Freischlag J, Bennion RS, Thompson JE Jr. Complications of diverticular disease of the colon in young people. Dis Colon Rectum. 1986. 29:639–643.
24. Ouriel K, Schwartz SI. Diverticular disease in the young patient. Surg Gynecol Obstet. 1983. 156:1–5.
25. Acosta JA, Grebenc ML, Doberneck RC, McCarthy JD, Fry DE. Colonic diverticular disease in patients 40 years old or younger. Am Surg. 1992. 58:605–607.
26. Minardi AJ Jr, Johnson LW, Sehon JK, Zibari GB, McDonald JC. Diverticulitis in the young patient. Am Surg. 2001. 67:458–461.
27. Chautems RC, Ambrosetti P, Ludwig A, Mermillod B, Morel P, Soravia C. Long term follow-up after first acute episode of sigmoid diverticulitis: is surgery mandatory?: a prospective study of 118 patients. Dis Colon Rectum. 2002. 45:962–966.
28. Spivak H, Weinrauch S, Harvey JC, Surick B, Ferstenberg H, Friedman I. Acute colonic diverticulitis in the young. Dis Colon Rectum. 1997. 40:570–574.
29. Cho KC, Morehouse HT, Alterman DD, Thornhill BA. Sigmoid diverticulitis: diagnostic role of CT: comparison with barium enema studies. Radiology. 1990. 176:111–115.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download