Abstract
We report a case of diabetic ketoacidosis (DKA) and hypertriglyceridemia (severely elevated to 15,240 mg/dL) complicated by acute pancreatitis, which was treated successfully with insulin therapy and conservative management. A 20-yr-old woman with a history of type 1 diabetes came to the emergency department 7 months after discontinuing insulin therapy. DKA, severe hypertriglyceridemia and acute pancreatitis were diagnosed, with DKA suspected of contributing to the development of the other conditions. In Korea, two cases of DKA-induced hypertriglyceridemia and 13 cases of hypertriglyceridemia-induced acute pancreatitis have been previously reported separately.
Diabetic ketoacidosis (DKA) is an acute metabolic complication that occurs mainly in type 1 diabetes mellitus (1). The risk factors are omission of insulin, infection, trauma and acute pancreatitis (2, 3). Insulin deficiency increase free fatty acid (FFA) and amino acids release from adipose tissue and muscle, respectively and increased counter-regulatory hormones causes increased gluconeogenesis and glycogenolysis in the liver (4, 5). Elevated FFA taken up by liver leads to increased production of very low density lipoprotein (VLDL), which causes hypertriglyceridemia (2-4). Hypertriglyceridemia is an uncommon cause of acute pancreatitis accounting for 1-4% of cases, especially when the serum triglyceride (TG) level exceeds 1,000 mg/dL (5). In Korea, 13 cases of hypertriglyceridemia-induced acute pancreatitis have been reported. In two cases, where hypertriglyceridemia was noted along with DKA, the serum TG levels were severely elevated (12,864 and 11,929 mg/dL) but it did not cause acute pancreatitis (6, 7) (Tables 1, 2). The present case also involved DKA and an extremely high TG level (15,240 mg/dL), which ultimately culminated in the development of acute pancreatitis. DKA, hypertriglyceridemia and acute pancreatitis were successfully resolved by insulin and hydration therapy.
A 20-yr-old female visited the emergency department because of a 1-day history of vomiting (10 times) and was experiencing epigastric pain with diarrhea on March 23, 2009. The upper gastric pain was continuous without radiation. The patient had been drinking almost daily alcoholic beverages soju (alcohol concentration in the range of 19-22%) for 5 days prior to admission. The patient had a smoking history of one pack-year. Two years previously, the patient experienced DKA accompanied by acute pancreatitis. At that time, the patient had been diagnosed with type 1 diabetes mellitus. Insulin treatment began at that time. However, 7 months prior to the current admission, the patient ceased taking insulin.
Upon admission, the patient was determined to be 161 cm in height, 55 kg in weight with a body mass index of 21.2. On admission, the patient was alert but appeared acutely ill. Initial vital signs were blood pressure 90/60 mmHg, pulse rate of 88 beats/min, respiratory rate of 20/min and body temperature of 36.5℃. Physical examination revealed a dehydrated tongue and skin turgor. There was no evidence of xanthoma, xanthelasma or eruptive xanthoma. No palpable lymph node enlargement was apparent on head and neck examination, and no abdominal tenderness on abdominal examination. Bowel sound was normoactive.
Initial laboratory findings were ABGA (pH 7.148, pCO2 12.9 mmHg, pO2 126 mmHg, HCO3- 8.4 mM/L, SaO2 98.0%), glucose level 281 mg/dL, hemoglobin A1c 13.8% , C-peptide (premeal) 0.441 ng/mL (normal reference: 1.1-4.4 ng/mL), total cholesterol 1,640 mg/dL, TG 15,240 mg/dL, measured low density lipoprotein cholesterol (LDL-C) 246 mg/dL (determined by homogeneous enzymatic colorimetry method assay), high density lipoprotein cholesterol (HDL-C) 69 mg/dL, serum ketone body 3.1 mM/L (normal reference 0-0.05 mM/L), total bilirubin 1.2 mg/dL, AST 19 IU/L, ALT 14 IU/L, total protein 8.4 g/dL, serum albumin 4.1 g/dL, alkaline phosphatase 147 IU/L, serum amylase 81 U/L, serum lipase 108 U/L, WBC 13,310/µL, hemoglobin 13.9 g/dL, hsCRP 5.616 mg/dL, BUN 14.0 mg/dL, creatinine 0.6 mg/dL, sodium 125 mEq/L, potassium 4.4 mEq/L and chloride 95 mEq/L. There were no abnormalities in the coagulation test. Serum sample was milky and turbid, which suggested a lipemic state (Fig. 1). Anti-glutamic acid decarboxylase (GAD) antibody was 0.12 U/mL (normal reference 0-0.9 U/mL), and anti-islet antibody-2 (IA-2) antibody was <0.4 U/mL (normal reference 0-0.4 U/mL). Apolipoprotein E genotyping assessed by polymerase chain reaction revealed ε2/ε3.
The patient was aggressively hydrated and treated with intravenous insulin in the intensive care unit. On admission, serum sodium was 125 mEq/L and serum osmolality was 317 mOsm/kg. We suspected pseudohyponatremia caused by hyperlipidemia and hyperglycemia, and tried normal saline infusion. In spite of significant improvement in glucose level for the first 12 hr, sodium level dropped to 115 mEq/L. After correcting the hyponatremia with 3% NaCl solution, continuous insulin infusion and hydration for next 12 hr, serum sodium level rose to 121 mEq/L.
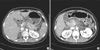
On the second day of hospitalization, the patient complained of aggravated epigastric pain. Follow-up laboratory analyses revealed a serum amylase level of 443 U/L and a serum lipase level of 615 U/L. On abdominal computed tomography (CT) scan, the pancreas was diffusely swollen with peripancreatic fat infiltration and fluid collection, which suggested acute pancreatitis grade D, according to the Balthazar CT severity index (Fig. 2). The Ranson's score was 4 at this point. There was no lipidemia retinalis on an ophthalmologic exam.
On the third day of hospitalization, the TG was 506 mg/dL, total cholesterol 281 mg/dL, LDL-C 101 mg/dL and HDL-C 36 mg/dL. The epigastric pain had diminished. The patient commenced oral intake, multiple subcutaneous insulin injection and fibrate medication.
On the fourth day of hospitalization, the serum amylase level was 50 U/L and lipase level was 36 U/L. The patient was discharged without any complication on post-admission day 8. Fourteen days after discharge, analyses revealed total cholesterol 308 mg/dL, TG 309 mg/dL, LDL-C 184 mg/dL, HDL-C 66 mg/dL, serum amylase level 107 U/L and serum lipase level 48 U/L. Lipoprotein electrophoresis performed after recovery showed a normal pattern.
In DKA, the deficiency of insulin activates lipolysis in adipose tissue releasing increased FFA, which accelerates formation of VLDL in the liver. In addition, reduced activity of lipoprotein lipase in peripheral tissue decreases removal of VLDL from the plasma, resulting in hypertriglyceridemia (8). Moderate hypertriglyceridemia is common during episodes of DKA (9). However, severe hypertriglyceridemia, which is defined as a TG level >2,000 mg/dL, is rare. Although morbidity is <1%, clinicians should be aware that devastating consequences such as acute pancreatitis or lipidemia retinalis are possible (8). In extreme cases, co-existence of genetic mutations in lipoprotein lipase should be suspected (10).
During treatment of DKA with severe hypertriglyceridemia, pseudohyponatremia or pseudonormoglycemia due to laboratory interference may lead to delay of proper management. Frier et al. suggested that if serum triglyceride concentration exceeds 2,500 mg/dL, measured electrolyte can decrease by over 5% because of the intracellular movement of serum lipid components. Therefore, in the hyponatremic state, the clinician should consider the possibility of pseudohyponatremia and avoid overtreatment with hypertonic saline (11). We presently observed a decreased sodium level of 125 mEq/L to 115 mEq/L during the recovery phase, which necessitate temporary correction with 3% hypertonic saline.
In severe hypertriglyceridemia, there is an increased risk of developing acute pancreatitis. The mechanism is related to high plasma chylomicrons or TGs, which are hydrolyzed by lipase in the pancreatic capillaries and subsequently trigger FFA release (12) that, in turn, causes activation of trypsinogen and commences pancreatic capillary damage by free radical damage (13, 14). The common clinical scenario of hypertriglyceridemia-induced acute pancreatitis involves poorly-controlled diabetes mellitus with type IV hyperlipidemia (5), or chronic alcoholism (15). In contrast, moderate hyperlipidemia (usually <400 mg/dL) can be observed secondary to acute pancreatitis and should not be confused with the marked hypertriglyceridemia that causes acute pancreatitis (16), as in the present case.
Of note, normoamylasemia is possible in about 50% of patients with hypertriglyceridemia-induced pancreatitis. The mechanism is believed to be the interference with in vitro determination of the actual amylase level by disturbance of the calorimetric method. Serial dilutions of the sample could reduce interference of light transmission by hyperlipidemic serum (17). In the present case, increased amylase occurred parallel to the decreased TG level, which might have delayed the diagnosis of acute pancreatitis at initial presentation.
Although acute pancreatitis can initiate DKA, DKA itself may mask a co-existing acute pancreatitis that occurs in 10-15% of cases due to ambiguous clinical presentations (16). Even worse, nonspecific elevations of amylase and/or lipase without clinical evidence of pancreatitis have been reported in 24.7-79.0% of DKA cases (17). At least in those patients with continuous abdominal pain, it is prudent to seek further laboratory evaluation or a CT scan of the abdomen. In our case, not surprisingly, because of the severe dehydration secondary to DKA, the initial and 48 h Ranson's score was 4 points each, which is relatively high. In a previous study, the clinical course of acute pancreatitis with DKA seemed to be mild, although the mean Ranson's score was 1.36±0.5 and the mean CT severity index (total 20 point) score was 4.6±1.2; none of the patients developed systemic complication (17).
In the present case, hypertriglyceridemia was controlled with insulin without lipid lowering agents. However, in severe hypertriglyceridemia, physicians should consider the application of plasma exchange to avoid complications (18). The present patient has since been administered fibrate, which is the first-line drug of hypertriglyceridemia, and insulin treatment, in order to prevent recurrence of pancreatitis (19).
To our knowledge, this is the first report of a case in Korea of DKA and severe hypertriglyceridemia, complicated by acute pancreatitis. Although moderate hypertriglyceridemia in DKA is common, if the TG level exceeds 1,000 mg/dL, the clinician should consider the devastating consequences such as acute pancreatitis or lipemic retinalis, which might benefited from insulin administration and conservative management, unless otherwise necessitating the plasma exchange.
Figures and Tables
Fig. 1
Photograph of hyperlipidemic serum extracted by centrifugation from the patient's blood sample.

Fig. 2
Contrast-enhanced pancreas CT scan (arterial phase). Initial pancreas CT scan shows diffuse swelling of pancreas body, tail, demonstrating CT grade D acute pancreatitis with peripancreatic fat infiltration (arrow), fluid collection suggesting inflammation (A) and edematous change of pancreatic head (openarrow) (B).
References
1. Kitabchi AE, Nyenwe EA. Hyperglycemic crises in diabetes mellitus: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Endocrinol Metab Clin North Am. 2006. 35:725–751.

2. Chiasson JL, Aris-Jilwan N, Belanger R, Bertrand S, Beauregard H, Ekoe JM, Fournier H, Havrankova J. Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar state. CMAJ. 2003. 168:859–866.
3. Kitabchi AE, Umpierrez GE, Murphy MB, Barrett EJ, Kreisberg RA, Malone JI, Wall BM. Hyperglycemic crises in diabetes. Diabetes Care. 2004. 27:Suppl 1. S94–S102.
4. Exton JH. Mechanisms of hormonal regulation of hepatic glucose metabolism. Diabetes Metab Rev. 1987. 3:163–183.

5. Fortson MR, Freedman SN, Webster PD 3rd. Clinical assessment of hyperlipidemic pancreatitis. Am J Gastroenterol. 1995. 90:2134–2139.
6. Choi DS, Oh JH, Park IB, Kim JW, Choi KM, Kim YH, Kim NH, Kim SJ, Baik SH. A case of severe hypertriglyceridemia with diabetic ketoacidosis. J Korean Diabetes Assoc. 1999. 23:715–721.
7. Choi SH, Sohn TS, Lee JI, Kim ES, Choi WH, Shin J, Son HS. A case of severe hypertriglyceridemia in diabetic ketoacidosis. Korean Clin Diabetes. 2008. 9:336–340.

8. Fulop M, Eder H. Severe hypertriglyceridemia in diabetic ketosis. Am J Med Sci. 1990. 300:361–365.

9. Fulop M, Eder HA. Plasma triglycerides and cholesterol in diabetic ketosis. Arch Intern Med. 1989. 149:1997–2002.

10. Karagianni C, Stabouli S, Roumeliotou K, Traeger-Synodinos J, Kavazarakis E, Gourgiotis D, Lambrou J, Kanavakis E. Severe hypertriglyceridaemia in diabetic ketoacidosis: clinical and genetic study. Diabet Med. 2004. 21:380–382.

11. Frier BM, Steer CR, Baird JD, Bloomfield S. Misleading plasma electrolytes in diabetic children with severe hyperlipidaemia. Arch Dis Child. 1980. 55:771–775.

12. Havel RJ. Pathogenesis, differentiation and management of hypertriglyceridemia. Adv Intern Med. 1969. 15:117–154.
14. Tsuang W, Navaneethan U, Ruiz L, Palascak JB, Gelrud A. Hypertriglyceridemic pancreatitis: presentation and management. Am J Gastroenterol. 2009. 104:984–991.

15. Yadav D, Pitchumoni CS. Issues in hyperlipidemic pancreatitis. J Clin Gastroenterol. 2003. 36:54–62.

16. Nair S, Yadav D, Pitchumoni CS. Association of diabetic ketoacidosis and acute pancreatitis: observations in 100 consecutive episodes of DKA. Am J Gastroenterol. 2000. 95:2795–2800.

17. Yadav D, Nair S, Norkus EP, Pitchumoni CS. Nonspecific hyperamylasemia and hyperlipasemia in diabetic ketoacidosis: incidence and correlation with biochemical abnormalities. Am J Gastroenterol. 2000. 95:3123–3128.

18. Furuya T, Komatsu M, Takahashi K, Hashimoto N, Hashizume T, Wajima N, Kubota M, Itoh S, Soeno T, Suzuki K, Enzan K, Matsuo S. Plasma exchange for hypertriglyceridemic acute necrotizing pancreatitis: report of two cases. Ther Apher. 2002. 6:454–458.
19. Huang DB, Raskin P. Diabetic hypertriglyceridemia-induced acute pancreatitis masquerading as biliary pancreatitis. J Diabetes Complications. 2002. 16:180–182.

20. Lee CY, Lee MK, Lee SY, Hong SN, Kim HH, Kang BH, Kang HW, Lee BW, Park YJ, Min YK, Lee MS, Kim KW, Kim JH. A case of acromegaly with diabetic detoacidosis and hypertriglyceridemia-induced acute pancreatitis. J Korean Soc Endocrinol. 2002. 17:110–116.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download