This article has been
cited by other articles in ScienceCentral.
Abstract
We investigated the availability of motor unit number estimation (MUNE) as a quantitative method to assess the severity and clinical progression of amyotrophic lateral sclerosis (ALS). The 143 ALS patients were evaluated by statistical MUNE and the revised amyotrophic lateral sclerosis functional rating scale (ALSFRS-R). By using mean values of MUNE according to disease duration, regression equation between mean MUNE and disease duration was presented as a formula. The individual MUNE ratio was calculated by dividing individual MUNE value by mean MUNE value. All patients were classified into 2 groups (MUNE ratio <1 vs. MUNE ratio ≥1) according to the MUNE ratio. Comparison between the 2 groups revealed that the patients in MUNE ratio <1 group or MUNE ratio ≥1 group were respectively assigned to rapid progression or slow progression. We recommended informative mean values of MUNE and best regression equation in ALS patients according to disease duration. These values allow us to evaluate the severity and rapidity of progression in ALS.
Keywords: Amyotrophic Lateral Sclerosis, Motor Unit Number Estimation
INTRODUCTION
Amyotrophic lateral sclerosis (ALS), the most common adult-onset motor-neuron disease, is pathologically characterized by progressive loss of the upper and lower motor neurons in the brainstem motor nuclei and the anterior horn of the spinal cord. This pattern of neurodegeneration produces progressive weakness, muscular wasting, and spasticity. The disease starts segmentally before it spreads and causes death from respiratory failure or infection a few years after its onset. However, the modalities of disease progression are diverse among patients (
1). Diversities of the disease course in ALS make it difficult to give patients accurate information about the progress of the disease and to ascertain whether there is a beneficial effect of the treatment to slow the progression of the disease. Nerve conduction study (NCS) and electromyography (EMG) have been used to diagnose ALS and are relatively accurate methods for diagnosing ALS (
2). But these methods do not reveal the severity of the disease and the rate of its progression. Therefore, a quantitative electrophysiological method is needed to monitor disease severity, rate of progression, and response to treatment. Motor unit number estimation (MUNE) can be a valuable tool for assessing ALS (
3-
5). MUNE is an electrophysiological technique that estimates the number of functioning motor units in a given muscle or group of muscles (
6,
7). MUNE is calculated by dividing the value of the maximal compound muscle action potential (CMAP) by the value of the average surface-recorded motor unit potential (SMUP) (
3-
8). Of the various MUNE techniques that essentially obtain representative samples of SMUPs in different ways, the statistical method has been reported to be more representative of the actual distributions of motor units than the results obtained using other MUNE methods (
8-
14). A few clinical trials have been performed in ALS patients and showed the reliability and reproducibility of statistical MUNE (
8-
11). But, so far, the MUNE has been limitedly demonstrated in the diagnosis of ALS and evaluation of the severity of the disease. When analyzing the results of MUNE in ALS patients, we are usually confounded about the clinical application of MUNE, e.g. which method, among the program determined MUNE (P-MUNE), number-weighted MUNE (NW-MUNE), and size-weighted MUNE (SW-MUNE), is clinically the most representative in ALS patients? How can we explain the results of MUNE to ALS patients?
To better address the availability of MUNE as a quantitative method for assessing the severity and clinical progression of the disease in patients with ALS, we investigated the mean values of MUNE according to the disease duration in a wide range of ALS patients, and compared the electrophysiological rapidity of the progression by the MUNE ratio with the clinical rapidity of progression by ALSFRS.
MATERIALS AND METHODS
Patients
Patients with definite or laboratory-supported probable ALS according to the revised El Escorial criteria were consecutively recruited from the ALS Clinic of the Seoul National University Hospital where the protocol was approved by the Institutional Review Board (H-1002-027-309) (
2). We excluded patients who had a history of brain injury, stroke, diabetes, ulnar neuropathy, polyneuropathy and no potential in the abductor digiti minimi (ADM) muscle, which was confirmed by NCS/EMG, clinical history, laboratory examinations and, occasionally imaging study. Finally, 143 patients were enrolled and were evaluated by MUNE in bilateral ADM muscles (
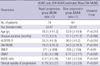
Table 1). The first manifestation of patients was respectively presented to be upper extremity weakness in 70 patients, lower extremity weakness in 37, and dysarthria or dysphagia in 36 (
Table 1). The mean disease duration was generally recorded by the patient's statement of the number of months after onset of the first symptoms. Functional impairment due to ALS was evaluated using the revised amyotrophic lateral sclerosis rating scale (ALSFRS-R), which consists of 12 items that measure bulbar, upper extremity, lower extremity, and respiratory functions (
15). The scores range from 0 (severe impairment) to 48 (normal functioning), with a low score indicating greater impairment.
Motor unit number estimation
MUNE was performed with a commercially available version of the statistical MUNE method (Nicolet Biomedical, Inc., Madison, WI, USA). Percutaneous stimulation of the ulnar nerve at the wrist was delivered by a flat bar electrode that was wrapped around the arm. The maximal compound muscle action potential (CMAP) of the ADM muscle was then recorded using surface electrodes. After obtaining a CMAP by supramaximal stimulation, a sigmoid curve of the CMAP area was obtained as a function of increased stimulus intensity by delivering 30 computer controlled stimuli at 1 Hz, which increased in intensity by equal increments from just sub-threshold to just supramaximal intensity. Three scan graphs were obtained sequentially by repeating the above procedure. The test areas of 4 segments were composed in most patients. The sum of the test areas accounted for 80% or more of the whole CMAP area in cases with 3 or fewer test areas. The test areas were selected according to the rules designed to ensure inclusion of the most neurogenically compensated segments, thus indicating the presence of abnormally large MUPs because of compensatory reinnervation. Initially, we selected areas either with a consistent area gap exceeding 10% of the maximal CMAP or with a gap exceeding 5% of the maximal CMAP that was flanked by more than 2 CMAP areas of the sample size on both the lower and upper sides in 3 consecutive scan graphs. On acquiring the first set of 30 submaximal CMAPs in the test area, each set of CMAPs was found to show a poisson distribution that skewed slightly to lower amplitude. Stimuli were delivered at 2 Hz, and subsequent sets of the 30 stimuli were delivered in the same manner until the amplitude of the SMUP obtained by each set showed a variation of less than 10%. SW-MUNE was calculated as proposed by Olney et al. (
12), and NW-MUNE as described by Shefner et al. (
13). However, it was the limitation of MUNE that MUNE was performed mainly in the hand muscle and therefore MUNE parameters might not reflect the overall functional status of ALS patients. To reduce this limitation, we performed MUNE in bilateral hand muscle, and used the average values in this study.
Statistical analysis
SPSS v17.0 was used for data analysis and frequency tables were generated for the demographic data, ALSFRS, and mean values of the individual MUNE (including P-MUNE, NW-MUNE, and SW-MUNE) (
Table 1). The correlations of the individual MUNE with disease duration and ALSFRS-R were respectively investigated by using the Pearson's correlation coefficient for samples, and they were confirmed to be normal distribution by using Kolmogorov-Smirov test before performing Pearson's correlation. According to the disease duration (up to 6, 12, 18, 24, 30, and 36 months), clinical characteristics, mean values of ALSFRS-R, individual MUNE, and rapidity of disease progression were presented (
Table 1) and best regression equation between disease duration and mean SW-MUNE was analyzed to predict mean SW-MUNE in any disease duration (Estimated mean SW-MUNE=48.03-0.85×[disease duration]). Four patients with disease duration over 36 months among 143 patients were excluded from this procedure. The MUNE ratios of patients were calculated by dividing the individual SW-MUNE value by estimated mean SW-MUNE using regression equation (MUNE ratio=measured SW-MUNE/estimated mean SW-MUNE using regression equation). Patients were classified into 2 groups according to the results of the MUNE ratio, i.e., (MUNE ratio <1) vs. (MUNE ratio ≥1). An assessment of the clinical rapidity of disease progression by ALSFRS-R was made as follows. Rapidity of progression=(48-ALSFRS-R)/disease duration (
16). The ALSFRS-R, SW-MUNE, CMAP, SW-SMUP, clinical rapidity of progression by ALSFRS-R, and MUNE ratio were compared between these 2 groups by using unpaired t-test. A significance level was set at
P<0.01.
RESULTS
We evaluated 143 patients and obtained 286 results of MUNE. All patients were evaluated in the bilateral ADM muscles. The age of the participants ranged from 20 to 79 yr (mean age: 55.1 yr, SD±11.1), and 89 participants (62.2%) were male (
Table 1). The mean disease duration, which is generally the patient's statement of the number of months after onset of the first symptoms, was 17.1 months (SD±10.0) (
Table 1). The mean score of ALSFRS-R in all ALS patients was 36.9 (SD±6.2). The individual mean values of P-MUNE, NW-MUNE, and SW-MUNE were 55.2 (SD±38.9), 41.3 (SD±26.3), and 33.2 (SD±24.2), respectively (
Table 1).
The individual value of MUNE and CMAP were found to correlate with disease duration and ALSFRS-R. Among them, S-MUNE was statistically the most significant (Pearson correlation efficient with disease duration and ALSFRS-R, respectively: -0.329 [
P<0.001]; 0.576 [
P<0.001]) (
Fig. 1). Similarly, these significant correlations were also detected when the age was considered the control variable.
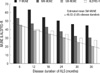
The mean values of the individual MUNE and ALSFRS-R according to the disease duration (up to 6, 12, 18, 24, 30, and 36 months) were also presented. The mean values of MUNE showed a gradually decreasing pattern as the disease duration increased (
Table 1,
Fig. 2). From mean disease duration and mean SW-MUNE, regression equation was analyzed (Estimated mean SW-MUNE=48.03-0.85×[disease duration]) (
Fig. 2) and MUNE ratio were calculated by dividing individual patient's SW-MUNE by estimated mean SW-MUNE using regression equation for the individual ALS patients. The MUNE ratio were statistically correlated with the clinical rapidity of disease progression by ALSFRS (Pearson's correlation coefficient=-0.449;
P<0.001) (
Fig. 1). Using the cut-off limit for the rapidity of progression by the MUNE ratio in ALS patients, 79 patients were assigned to the rapid progression group (MUNE ratio <1). The remaining 64 patients were assigned to the slow progression group (MUNE ratio ≥1) (
Table 2). The mean MUNE ratio was 0.47 (SD±0.26) in the rapid progression group and 1.67 (SD±0.53) in the slow progression group (
Table 2). The clinical rapidity of progression by ALSFRS-R, SW-MUNE, ALSFRS-R, MUNE ratio and CMAP were compared between the 2 groups, and significant differences were detected by using unpaired t-test. The electrophysiological rapidity of progression by MUNE ratio was coincident with the difference of CMAP and the clinical rapidity of progression by ALSFRS (
Fig. 1,
Table 2).
DISCUSSION
The diagnosis of ALS is usually made by clinical manifestations and NCS/EMG, which can provide accurate diagnostic information on the widespread denervation potentials (
17). However, the quantification of the disease severity is not easy (
2). The loss of motor unit in ALS is gradually progressive as the disease advances. Therefore, we can evaluate and quantify the disease progression through the number of motor units (
3,
4). MUNE is the number of functioning motor units in a given muscle or group of muscles that can be used to monitor disease progression or severity of disease in ALS (
3,
4). A few longitudinal studies using MUNE in some ALS patients have been reported and suggested that MUNE decreases as the disease progresses and that MUNE is a very reliable and reproducible method in ALS (
10-
14). However, there has been no document on mean values of MUNE according to the disease duration in a wide range of ALS patients. This study demonstrates that statistical MUNE highly correlates with disease duration and ALSFRS-R. The clinical correlation can be improved by applying a size- or number-weighted modification. Among all modalities of MUNE and CMAP, SW-MUNE showed the most significant correlation with disease duration and ALSFRS-R. The mean values of SW-MUNE showed most significantly decreasing patterns according to disease duration, therefore, the study also shows that SW-MUNE is more practicable than P-MUNE, NW-MUNE or CMAP, even though CMAP, which is a relatively simple measurement, also decreased significantly over disease course.
It is most suggestive that this study presented mean values of MUNE according to disease duration, since it improved the practicability of MUNE. After individual evaluation of the ALS patients by MUNE, these results can be compared with the mean values of the large number of ALS patients presented in this study. If the result of the patient's MUNE was greater than the mean value of all ALS patients with the equivalent disease duration, it could be supposed that the patient has a more favorable course compared with the average of all ALS patients. Conversely, if the result of the patient's MUNE was lower than the mean value of all ALS patients with the equivalent disease duration, it could be supposed that the patient has a faster disease progression than the average of all ALS patients. We assigned all patients to either the rapid (MUNE ratio <1) or slow progression group (MUNE ratio ≥1) on the basis of the MUNE ratio of SW-MUNE. The results (
Fig. 1,
Table 2) revealed that the MUNE ratio of SW-MUNE was coincident with the clinical rapidity of disease progression by ALSFRS, and the statistical differences were showed in CMAP, ALSFRS-R and MUNE ratio. Therefore, supposition of the rapidity of disease progression or severity of the disease in ALS patients is possible by comparing the results of individual MUNE with the estimated mean MUNE according to the disease duration.
The limitation of the present study is that MUNE of patients was performed in only ADM muscle, and so the results may be restricted to hand function in ALS and we cannot conclusively assert that the same MUNE value in different patients represents the same disease course or severity in patients. However the ALS is generalized disease simultaneously involving upper extremity, lower extremity and cranial muscle, therefore hand muscle weakness can represent the severity of generalized weakness to some degree. Of course, further studies over multiplicate MUNE, which measures upper extremity, lower extremity and cranial muscle in ALS patients, are required in the future.
In conclusion, the mean values of MUNE and regression equation assessed in a wide range of ALS patients are very informative in that the results can be compared with MUNE of individual ALS patient, which allows physicians to estimate the disease severity and rapidity of progression in ALS patients. And its combination with other measurements, such as CMAP or clinical rapidity of ALSFRS-R, may enhance overall prognostic accuracy in ALS.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download